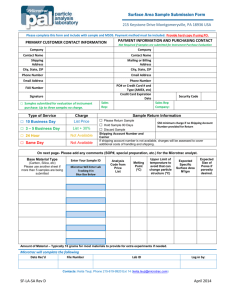
Department of Physiology Safety & Health
advertisement

Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 1 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Prepared/Review By Approved By Next Review Date Oh Chu Yun Dr. Leung Pui Lam Bernard 16/6/2013 * Review Date = Future date for the next revision (every 3yrs) 1.0 OBJECTIVE The purpose of this procedure is to detail how biological materials are to be safely transferred or transported in National University of Singapore (NUS) to ensure they are in compliance with all international or local regulations. 2.0 SCOPE 2.1 This procedure covers the transport of biological materials in or outside of NUS and the transfer of biological materials within NUS. 2.2 This procedure is to be followed when transporting or transferring 2.2.1 Any First Schedule biological agent 2.2.2 Any Second Schedule biological agent 2.2.3 Any Third Schedule biological agent transported in quantities aggregating 10 liters or more on any conveyance at any one time 2.2.4 Any Fifth Schedule toxin 3.0 DEFINITIONS 3.1 In this procedure, transport refers to the shipment of biological agents across national boundaries by a commercial conveyance. 3.2 In this procedure, transfer refers to the movement of biological agents between laboratories, within buildings or between research institutes/departments. 3.3 Dangerous Goods Regulations (DGR) is regulated by the International Air Transport Association (IATA). These regulations provide packaging and labeling requirements for infectious substances and materials, as well as clinical specimens that have a low probability of containing an infectious substance. These are the regulations followed by Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 2 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 the airlines. These regulations are derived from the Committee of Experts on the Transport of Dangerous Goods, United Nations (UN) Secretariat, and the Technical Instructions for the Transport of Dangerous Goods by air which is provided by the International Civil Aviation Organization (ICAO). 3.4 The Exporter/Shipper/Consignor are the party responsible for preparing the materials for transport. 3.5 The Shipper/Carrier are the party responsible for transporting the materials from the consignor to the consignee. 3.6 The Importer/Consignee is the person or organization (laboratory, company) receiving the materials. 3.7 Definitions of commonly transported materials such as diagnostic specimens, infectious substances, miscellaneous dangerous goods, biological products and toxic substances are found in Appendix 1. 4.0 RESPONSIBILITIES 4.1 The Director/HOD/PI of the lab performing the transfer/transport has overall responsibility for ensuring a system is established for the safe transfer or transport of biological materials. 4.2 The PI shall hold the responsibilities of the exporter for the transport of biological materials under this supervision. 4.3 The Consignor’s/Shipper’s/Exporter’s responsibilities are 4.3.1 To classify if a material is a dangerous good or not 4.3.2 To select a proper shipping name for the material 4.3.3 To ensure the proper packing and packaging material is used 4.3.4 To ensure there is proper marking/signage and labeling on the package 4.3.5 To arrange for the documentation 4.3.6 To make arrangements for notifying the importer 4.3.7 To update inventory records to reflect transfer of ownership of the materials 4.3.8 To be contactable at all times by the carrier or the importer 4.4 The Carrier’s responsibilities are 4.4.1 To ensure compliance with IATA requirements 4.4.2 To ensure proper packaging, storage, loading and transport of the container Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 3 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 4.4.3 To deliver the package and copy of the shipper’s declaration to the consignee 4.4.4 To inform the importers in times of delivery failure 4.5 The Importer’s/Consignee’s responsibilities are 4.5.1 To inspect packages and report back to shipper 4.5.2 To ensure exporters provide proper packaging and labeling 4.5.3 To arrange for the most timely and efficient collection on arrival 4.5.4 To maintain a copy of the shipper’s declaration 4.5.5 To obtain import and/or other permits 4.5.6 To update inventory records to reflect receipt of the materials 4.5.7 To keep track of the distribution of the human pathogen locally 4.5.8 To ensure human pathogens are appropriately disposed of after use 5.0 PROCEDURES 5.1 Transport: shipping across international borders Shipment of materials of biological origin is regulated locally by government agencies such as the Ministry of Health and Agri-Food and Veterinary Authority. 5.2 The import of infectious agents is governed by the Biological Agents and Toxins Act (Act 36 of 2005). MOH’s “Guidelines on the import, transport, handling and disposal of human pathogens for diagnosis, scientific research and industrial uses in Singapore” (online:http://www.moh.gov.sg/corp/publications/details.do?cid=pub_grps_service&id=13 991953) are to be specifically followed for the import of human pathogens. 5.3 The regulations of the importing country, which may involve applying for permits from their safety, health and environmental agencies, are also to be complied with. 5.4 Generally carriers would require you to follow International Air Transport Association (IATA) – Dangerous Goods Regulations (DGR) requirements. They would have specific requirements for different classes of materials. 5.5 Therefore the first step in transporting materials of biological origin is to determine their classification according to IATA requirements. The definitions of the different classifications are given in Appendix 2. 5.6 Transport regulations and requirements are continually being updated by carriers and government agencies. It is therefore recommended that the principal investigator check with all relevant agencies before shipping any regulated material. Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 4 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 5.7 Packaging The basic component of all shipping requirements, with various minor modifications, is triple packaging, as follows: 5.7.1 A primary container that contains the specimen and its sample container. This must be leak proof and tightly closed. A positive seal, such as parafilm or tape around the cap is needed; 5.7.2 A secondary container that contains the primary container and packaging capable of absorbing the specimen. This must be watertight, leak proof, puncture proof, and certified according to UN 6.2 packaging standards; 5.7.3 An outer rigid shipping container that contains the secondary container and other packing material. Strong cardboard embossed or printed with the UN 6.2 certification seal may be used. The shipping container must be able to withstand leakage of contents, shocks, pressure changes and other conditions encountered during normal transportation handling. The contents should not leak to the outside of the shipping container even if the primary container should leak, unless there is severe damage to the outer shipping container. 5.7.4 When shipping with dry ice, the dry ice must be outside the outer container. The outer container and the dry ice can be enclosed in a Styrofoam box with a sturdy cardboard outer cover. Shipping companies may not accept Styrofoam boxes as outer layers. These final layers of Styrofoam and cardboard are called the "over pack". 5.7.5 Absorbent materials such as paper towels, vermiculite, disposable diapers, miracle polymers, etc. Ensure there is enough absorbent to contain the entire sample should a leak occur. 5.7.6 Itemized contents list listing whatever is in the secondary container by full name and volume, for e.g. Human Cornovirus, 5 tubes @ 1.5 ml - 7.5 ml total volume. This list shall be placed between the secondary container and the outer packaging and provide advice for anyone who may need to open the box of its contents (e.g. Inspectors). Should the box be opened, they will use this list to aid in hazard cleanup. 5.8 Labeling/package marking The outer package must be clearly marked with the following: 5.8.1 "This End Up" indications to illustrate correct vertical orientation with the UN 6.2 certification seal embossed or printed. 5.8.2 The diamond-shaped “Infectious Substance – Hazard Class 6” shipping label (Appendix 6). There are size regulations for the label - at least 4 inches on each side. The label must be displayed prominently, all on one side of the box; it cannot wrap around a side or be hidden in a corner. Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 5 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 5.8.3 The proper shipping name, the UN identification number and the technical name of the agent. This must be on the outside of the box adjacent to, above or just below the Diamond Shaped 'Infectious Substance' label. i. There are only two choices for proper shipping name: a. infectious substance, affecting humans, or b. Infectious substance, affecting animals. In both cases, the comma between substance and affecting is a required part of the proper shipping name. ii. If the agent is infectious to both humans and animals, it is an infectious substance, affecting humans. The UN identification number is either UN 2814 for infectious substance, affecting humans or UN 2900 for infectious substance, affecting animals. The technical name of the agent is the proper species or virus name. It should be enclosed in brackets. 5.8.4 The name and telephone number of the person shipping the material. 5.8.5 The name and telephone number of the person receiving the package. 5.8.6 A 24-hour telephone number to be called in case of damage or leakage 5.8.7 A label stating the words, "inner packages comply to prescribed specifications" 5.8.8 If shipping with dry ice, a label showing UN 1845 and the weight of the dry ice present. Except for the UN 6.2 certification, all of the above must also be on the outer package (Over pack) if one is used. If dry ice is used in the shipment, the over pack must also bear a Class 9 diamond shaped sticker for dry ice (Appendix 6). Note: an over pack used for shipping infectious substances must not contain any other goods except the refrigerant and the related goods. Refer to Appendix 1 for details to be filled in the shipper’s declaration 5.9 Transfer: Exchange of materials between facilities When moving infectious substances or toxins between labs or buildings on campus or locations within the national boundary, the following procedures must be followed as a minimum: 5.9.1 The sample must be in a sealed primary container. Plastic containers may be used whenever possible. 5.9.2 The primary container should be placed in a sealed secondary container, with absorbent (e.g. paper towels) between primary and secondary container, of an Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 6 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 adequate amount suitable for the volume of sample transported. The infectious substances (class 6) sign must be affixed to the primary receptacle. 5.9.3 The secondary container must be affixed with an itemized list of contents of each primary receptacle placed in the secondary receptacle. 5.9.4 If dry ice is needed, the secondary container should be placed in an outer tertiary container, with the dry ice placed between the secondary and tertiary container. Never place dry ice in a sealed container. 5.9.5 The rigid outer container must be labeled with the following: 5.9.5.1 The biohazard (infectious substances class 6) label, or the toxin label, depending on the contents of the container (Appendix 6). 5.9.5.2 A statement that the container contains an infectious substance, or a toxin 5.9.5.3 Name of the agent 5.9.5.4 Name, address and phone number of the transferring lab 5.9.5.5 Name, address and phone number of the transferee 5.9.5.6 A 24-hour emergency phone number manned by someone with knowledge of hazards associated with the agent being transported 5.9.6 If the transfer process also involves ownership issues, there must be adequate documentation to acknowledge receipt of the materials. 5.9.7 Transfer of materials must be documented in the inventory records of both the consignor and consignee. 5.10 Training Training is mandatory for those involved in the offering, preparing for transport, packaging or transport of biological agents. 6.0 RECORDS Records of the following are to be kept for 3 years: 6.1 The facility/institution’s biosafety and security plan; 6.2 Current list of individuals with access to select agents for purpose of transfer/transport; 6.3 Particulars of individuals transferring and receiving biological agents 6.4 Training records for individuals with access to select agents for purpose of transfer/transport; 6.5 Accurate and current inventory records for lab/facility performing transfer/transport; 6.6 Permits and transfer documents; 6.7 Security records (e.g., transactions from automated access control systems, testing and maintenance of security systems, visitor logs); 6.8 Biosafety, containment, and security incident reports Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 7 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 7.0 REFERENCES a. Guidelines on the import, transport, handling and disposal of human pathogens for diagnosis, scientific research and industrial uses in Singapore, maintained by MOH b. Infectious Diseases Act (Cap. 137), regulated by MOH c. Dangerous Goods Regulations, International Air Transport Association (IATA) d. NSCU Biosafety Manual – Transportation and transfer of biological agents (online: http://www.ncsu.edu/ehs/www99/left/bioSafe/BioAppA.html#a3) Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 8 of 15 Safety & Health Laboratory (Location): 8.0 APPENDICES Cytokine biology Lab, MD9 Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 9 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Appendix 2 Classifications Dangerous substances are classified as follows irrespective of carrier: Class Meaning I. Diagnostic Specimens: a diagnostic specimen is defined as "any human or animal material including excreta, secreta, blood and its components, tissue, and tissue fluids being transported for diagnostic or investigational purposes, but excluding live infected humans or animals." In general, diagnostic specimens resulting from medical practice and research are not considered a public health threat. Classification of biological products and diagnostic specimens are compartmentalized into separate categories. Diagnostic specimens are divided into the following groups (in IATA DGR 3.6.2.4): a. those known or reasonably expected to contain pathogens in Risk Groups 2,3, or 4 and those where a relatively low probability exists that pathogens of Risk Group 4 are present. Specimens transported for the purposes of initial or confirmatory testing for the presence of pathogens fall within this group. b. those where a relatively low probability exists that pathogens of Risk Group 2 or 3 are present. Specimens transported for the purpose of routine screening tests or initial diagnosis for other than the presence of pathogens fall within this group. c. those known not to contain pathogens (not restricted) Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 10 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 II. Infectious substance: a material known to contain or suspected of containing a pathogen (including bacteria, viruses, rickettsia, parasites, fungi, recombinant hybrid or mutant microorganisms) that causes disease in humans or animals. An infectious substance must be assigned a risk group. III. Miscellaneous Dangerous Goods: substances which present dangers not covered elsewhere. Examples: asbestos; dry ice (solid carbon dioxide); magnetised material with a magnetic field strengthh of 0.159 A/m or more at a distance of 2.1m from the outside of the parcel. IV. Biological products. These products are derived from living organisms that are manufactured and distributed in accordance with the requirements of national governmental authorities that may have special licensing requirements. The products are used either for prevention, treatment, or diagnosis of disease in humans or animals, or for development, experimental or investigational purposes related thereto. They include, but are not limited to, finished or unfinished products such as vaccines and diagnostic products. Biological products are divided into the following groups (in IATA DGR 3.6.2.3): a. those which contain pathogens in Risk Group 1: those which contain pathogens under such conditions that their ability to produce disease is very low to none; and those known not to contain pathogens. Substances in this group are not considered infectious substances for the purposes of these regulations. b. those manufactured and packaged in accordance with the requirements of national governmental health authorities and transported for the purposes of final packaging or distribution, and use for personal health care by medical professionals or individuals. c. those known or reasonably expected to contain pathogens in Risk Groups 2, 3, or 4 and which do not meet the criteria of IATA DGR 3.6.2.3(b). Substances in this group must be classified in Division 6.2 under UN 2814 or UN 2900 as appropriate. V. Toxic substance. A material, other than a gas, that is known to be so toxic to humans as to cause death, injury, or harm to health if swallowed, inhaled, or contacted by the skin. Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 11 of 15 Safety & Health Laboratory (Location): Appendix 3. Appendix 4 Cytokine biology Lab, MD9 Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 12 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Appendix 5 Packing methods Packing and labeling of infectious substances Packing and labeling of clinical/diagnostic specimens Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 13 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Appendix 6. Labels This end up label. Infectious substance label (Class 6). The label shown above has a bright yellow background with black print. Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 14 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Toxin label. The label above has a white background with a black symbol and inscription. Biohazard clinical specimens label. The clinical specimen label shown above has a bright orange background with black print and is a rectangle measuring 2 inches high by 4 inches long. Department of Physiology Procedure No: PHY/SOP/RE04 Revision No: 001 Title: Effective Date : 16/6/2010 Transport & Transfer of Biological Agents (Adopted from OSHE/SOP/BS/02) Page: Page 15 of 15 Safety & Health Laboratory (Location): Cytokine biology Lab, MD9 Infectious substances shipped on dry ice (Class 9) label. For infectious substances shipped on dry ice two labels are required in addition to the infectious substance label, the class 9 label (also 100 cm. square) indicating a miscellaneous hazardous materials, and a label indicating the quantity of dry ice.