Supporting Information_20130711_SPark
advertisement

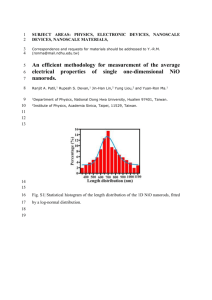
Single Inorganic-Organic Hybrid Photovoltaic Nanorod Sang-Hoon Yoo,1 Lichun Liu,5 Tea-Woong Ku,3 Soonchang Hong,1 Dongmok Whang2,3 * and Sungho Park1,4 * 1 Department of Chemistry, BK21 School of Chemical Materials Science, Sungkyunkwan University, Suwon 440-746, South Korea 2 School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon 440-746, South Korea 3 SKKU Advanced Institute of Nanotechnology, Sungkyunkwan University, Suwon 440-746, South Korea 4 Department of Energy Science, Sungkyunkwan University, Suwon 440-746, South Korea 5 College of Biological Chemical Science and Engineering, Jiaxing University, Jiaxing 314001, PR China *: Author to whom correspondence should be addressed. Electronic mail: dwhang@skku.edu (Dongmok Whang), spark72@skku.edu (Sungho Park) 1. Chemicals and Methodologies All chemicals were purchased from Sigma-Aldrich and were used as received if not specifically noted. All aqueous solutions were prepared using triply distilled deionized water (Millipore, 18.2 M). Alumina membranes (AAO, d = 25 mm, pore size = 200 nm) were purchased from Whatman International Ltd (England). Ag plating solution (1025 RTU) and Au plating solution (Orotemp 24 RTU) were purchased from Technic Inc. All electrochemistry related operations were performed on an Autolab equipped with a three electrode system. a. Ag nanoparticles synthesis The method for Ag nanoparticle synthesis has been described elsewhere. Briefly, 0.4 mg of silver oxide (Ag2O) dispersed in 500 mL of deionized water in a three-neck round bottom flask was heated to and maintained at 80 ℃. Once the temperature had equilibrated, H2 gas was slowly released into the flask to fill the head space above the liquid. Transition of the intial dark black color of the solution to a light yellow color (ca. 5 h) indicated the endpoint of the chemical reduction. Subsequently, the solution was cooled to room temperature, and the colloidal solution was filtered through filter paper three times with the aid of a vacuum pump to remove any Ag2O residue. Finally, the diameter of synthesized Ag NPs was evaluated to be ca. 125 nm. b. Preparation of back conducting layer on AAO Ag NPs immobilized on one side of the AAO template as an electrical conducting layer was performed by filtering 5 mL of the above synthesized colloidal solution into the AAO membrane. Further heating for 10 min in an 80 ℃ oven served to dry and consolidate the NP layer. c. Efficiency calculation The fill factor (FF), a ratio between maximum power output and the product of Isc and FF Voc, is commonly used to evaluate the quality of a PV device. I m Vm P max IscVoc IscVoc , Isc and Voc are short circuit current and open circuit voltage, respectively. Power conversion η efficiency () was FF ISC VOC Pmax light intensity light intensity . calculated from following equation 2. Figures Fig. S1 I-V curves of coxial structures with (a) and without (b) Au porous nanorod devices which exhibit diode behavior at dark condition. Fig. S2. I-V curves of pure Au nanorod (a) showing Ohmic behavior, CdSe nanorods (b) showing the deviation from Ohmic behavior which is typical response of semiconducting materials, and Ppy nanorods (c). Measurements were carried out under room temperature.