BAAS Application
advertisement
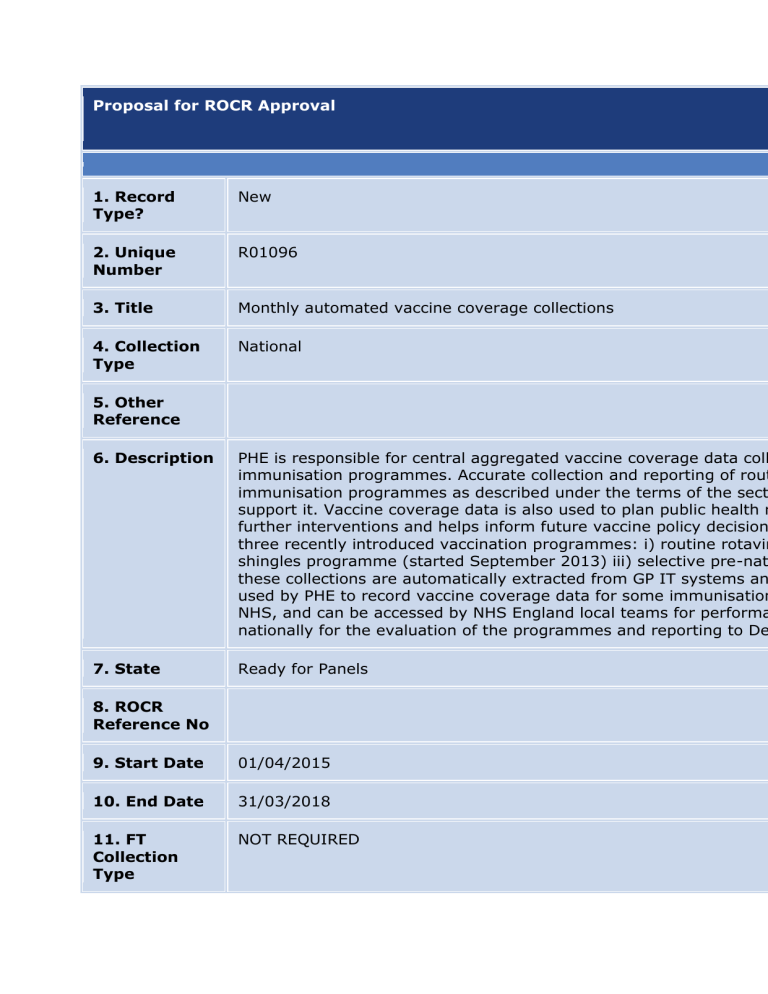
Proposal for ROCR Approval 1. Record Type? New 2. Unique Number R01096 3. Title Monthly automated vaccine coverage collections 4. Collection Type National 5. Other Reference 6. Description PHE is responsible for central aggregated vaccine coverage data coll immunisation programmes. Accurate collection and reporting of rout immunisation programmes as described under the terms of the sect support it. Vaccine coverage data is also used to plan public health r further interventions and helps inform future vaccine policy decision three recently introduced vaccination programmes: i) routine rotavir shingles programme (started September 2013) iii) selective pre-nat these collections are automatically extracted from GP IT systems an used by PHE to record vaccine coverage data for some immunisation NHS, and can be accessed by NHS England local teams for performa nationally for the evaluation of the programmes and reporting to De 7. State Ready for Panels 8. ROCR Reference No 9. Start Date 01/04/2015 10. End Date 31/03/2018 11. FT Collection Type NOT REQUIRED Proposal for ROCR Approval 12. Collection Type MANDATORY 13. Owning Organisation Public Health England 14. Owning Department Immunisation 15. Owner Name and Contact Details Name: Joanne White Email: joanne.white@phe.gov.uk Tel No: 02083277446 Location: Immunisation, Hepatitis and Blood Safety Department, Ce Health England, 61 Colindale Avenue, LONDON 16. Senior Supporting Official Name Dr Mary Ramsay 17. Senior Supporting Official Contact Details Title: Consultant Epidemiologist and Head of Immunisation, Hepatiti 18. Data Provider Burden Days 0 18. Data Provider Burden £ £0 18. Frequency Monthly Location: Immunisation, Hepatitis and Blood Safety Department, Ce Health England, 61 Colindale Avenue, LONDON Proposal for ROCR Approval 18. Source Organisations (Number of orgs) GP Practice (7810) 19. Set Up Costs £0 20. Other Costs £ 151571 21. Total Costs £ 151571 22. Please explain the reason for any increase or decrease in burden and provide details of the any other costs figure provided in Q20 There is no burden for these collections for the source organisations permission to participate in the collections survey data are automati to the ImmForm website in line with agreed survey-specific timetabl work, project management and development and testing of the Imm to develop and test a new clinical specification for a collection with G surveys where appropriate; Staff costs (admin/scientific/medical) 23. Benefits to Patients and the NHS The ability to reliably measure vaccine coverage plays an essential r identifying susceptible populations for further interventions and infor an accurate estimate for the eligible population (denominator) and a individuals who have received a particular vaccine dose (numerator) systems in England monitor immunisation coverage data for selectiv gender and ethnicity are available for most surveys. This information improve coverage, to detect inequalities and changes in vaccine cov have been given in a timely manner and promotes equal access to c These data are also used by the MHRA and in combination with data 24. Financial benefits to Maintaining high vaccine coverage in all areas will prevent outbreaks burden. The financial benefits from a societal perspective include the Proposal for ROCR Approval running this collection gained, and from an NHS perspective include savings to the NHS su 25. Publication methods Reports and local level data are published on the PHE website data 26. Publication Links https://www.gov.uk/government/collections/vaccine-uptake 27. Requesting Organisation Public Health England 28. Collection Method Web based collection 29. NHS Mandate Commitment Monitoring of the national immunisation programmes is mandated u service specifications that support it 30. Changes since last assessment 31. Data in operational systems Yes 32. Plans for collecting this data from operational systems PHE has an established work programme for vaccine coverage collec system suppliers on automated data extractions that are uploaded o 33. If the data was not collected, It would not be possible to monitor new and/or selective vaccination identify susceptible populations for further interventions and inform Proposal for ROCR Approval what would the consequences be 34. Is there an impact assessment or business case for this collection? If so please attach 35. Process required for others to go through to obtain the data Data are published in excel or tabular format on PHE website where time by NHS England local teams for performance management of th 36. Keywords coverage, vaccine, immunisation 37. National / Official statistic NA 38. Method used to store the data 39. Why sampling is not used Vaccine coverage is required to be monitored by every area of the c outbreaks of vaccine preventable disease could occur 40. Details of any pilots Surveys based on previous experience of using web based ImmForm 41. Equalities Age/Date of Birth, Gender, Ethnicity (NHS standard 16 + 1) Proposal for ROCR Approval dimensions used in the collection 42. Policy that the collection supports National immunisation programmes are routinely reviewed and revis meetings of the Joint Committee on Vaccination and Immunisation ( consideration of scientific and other evidence, used by DH to inform, https://www.gov.uk/government/uploads/system/uploads/attachme Minutes Jun 2014 meeting (Pre-natal pertussis) https://www.gov.uk immunisation#minutes; https://www.gov.uk/government/publicatio 43. IG Data type Aggregate










