GFM calcs summary
advertisement
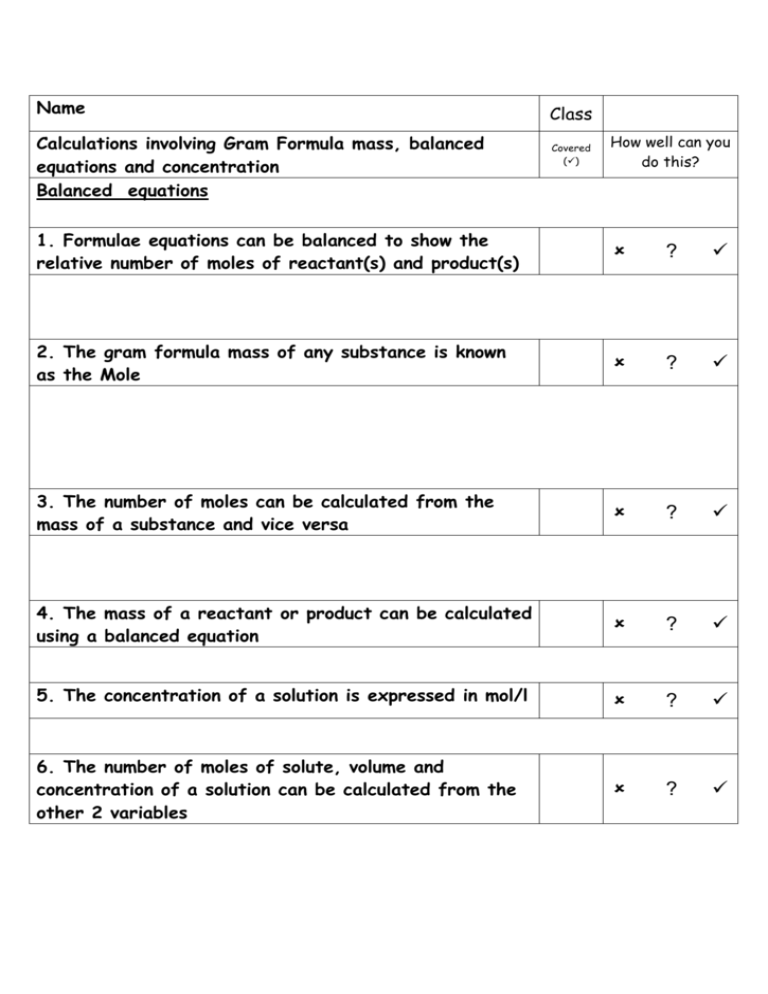
Name Calculations involving Gram Formula mass, balanced equations and concentration Balanced equations Class Covered () How well can you do this? 1. Formulae equations can be balanced to show the relative number of moles of reactant(s) and product(s) ? 2. The gram formula mass of any substance is known as the Mole ? 3. The number of moles can be calculated from the mass of a substance and vice versa ? 4. The mass of a reactant or product can be calculated using a balanced equation ? 5. The concentration of a solution is expressed in mol/l ? 6. The number of moles of solute, volume and concentration of a solution can be calculated from the other 2 variables ? Formula Mass (1) Calculate the formula mass of magnesium hydroxide. Step 1 Write the formula of the compound. Mg (OH) Mg(OH)2 2 1 Step 2 Work out the number of atoms of each element in the Formula and multiply by their relative atomic mass. 1Mg = 1 x 24.5 = 24.5 2O = 2 x 16 = 32 2H = 2x1 =2 Step3 Add the answer of the multiplication’s together. 24.5 + 32 + 2 = 58.5 a.m.u. ( a.m.u. = Atomic Mass Units) Mole Calculations (Mole = the gram formula mass or gfm) Calculate the mass of (i) 1 mole of copper (I) oxide (ii) 3 moles of calcium carbonate (i) Follow the steps shown above but replace a.m.u. with g. Cu O = Cu2O 1 2 2Cu = 2 x 63.5 = 127 1O = 1 x 16 = 16 127 + 16 = 143g The mass of 1 mole of copper (I) oxide is 143g. (ii) Step 1 Calculate the mass of 1 mole as shown. Ca (CO3) = Ca(CO3) 2 2 1Ca = 1 x 40 = 40 1C = 1 x 12 = 12 3O = 3 x 16 = 48 40 + 12 + 48 = 100g The mass of one mole is 100g. Step 2 Multiply the mass of 1 mole by the number of moles given in the question. 1 mole = 100g 3 moles = 100 x 3 = 300g The mass of 3 moles of calcium carbonate is 300g. Calculate the number of moles in 40g of calcium carbonate. Step 1 Calculate the mass of 1 mole. This method is shown above. Step 2 To calculate the number of moles in 40g we have to use the following equation. Number of moles = mass/mass of 1 mole (gfm) which can be put into a triangle m= mass in grams n = number of moles gfm= mass of 1 mole m n gfm Number of moles = 40g/100g = 0.4 40g of calcium carbonate contains 0.4 moles. Balancing equations We have already learned to write formula equations but these do not help chemists understand how much of the reactant is required to produce a certain amount of product. This is achieved by balancing equations. A balanced formula equation will have equal numbers of each element on both sides of an equation. This is achieved by putting numbers in front of the formulae. The number will multiply every element in that formula. Balance the following equations Mg + HCl MgCl2 + H2 In the above equation the Mg is balanced as we have the same number on both sides. We now have to balance the other elements. As there are two chlorines and two hydrogens on the right hand side we must multiply the HCl by 2. The balanced equation is shown below. Mg + CH4 + 2HCl MgCl2 + H2 O2 CO2 + H2 0 When balancing the above equation it helps if you balance C, then H and then finally O. The carbons are balanced, we have 4 H’s on the left and 2 on the right therefore we need to put a 2 in front of H2O to change the hydrogens into 4. CH4 + O2 CO2 + 2H20 This now gives us a total of 4 O’s on the right and only two on the left. So again we need to multiply the O2 by 2. CH4 + 2O2 CO2 + 2H20 Fractions can also be used to balance equations however this is not necessary as all equations can be balanced using whole numbers as shown in the example below. Mg + 1/2O2 MgO O2 2MgO Or 2Mg + Balance the following equations C2H6 + O2 CO2 + H 2O NaOH + H2SO4 Na2SO4 + H 2O C2H5OH + O2 CO2 + H 2O Fe2O3 + CO Fe + CO2 Calculations From Balanced Equations Calculate the mass of ammonia produced when 24g of nitrogen reacts with excess hydrogen. Write the balanced equation. N2 + 3H2 2NH3 Write down the number of moles of each compound under the formula, this is the number that is in front of the compounds formula. Once this has been done highlight the two compounds needed from the question. N2 + 1 mole 3H2 3 moles 2NH3 2 moles Write down the ratio between the highlighted compounds. 1 mole N2 2 moles NH3 Work out the gfm of each substance and then multiply by the number of moles. N2 28g NH3 17 x 2 = 34g (2 Moles) You will be given the mass of one of the compounds in the question. Change this down to one. In this example you are given the mass of nitrogen so divide both sides by 28 and then multiply by 24. 1g 34/28g We then multiply through by the mass given in the question. In this example it is 24 g. 24g (34/28) x 24 = 19.14g 24g of nitrogen will produce 19.14g of ammonia when hydrogen is in excess. (If a compound is in excess ignore that compound in the calculation) Example 2 Calculate the mass of methane that produces 9Kg of water when burned completely in air. CH4 + 2O2 1 mole CH4 CO2 + 2H2O 2 moles H2O 16 18 x 2 = 36 16/36 1 (16/36) x 9 9 = 4 kg When carrying out these types of calculations the units in the final answer will be the same as in the question, no conversions are needed. Follow the procedures shown to calculate the mass of hydrogen produced when 7.3g of HCl reacts with magnesium. Mg + 2HCl MgCl2 + H2 Concentration Calculations Calculate the concentration of 200 cm3 NaOH solution containing 2 moles of the compound. Step 1 Write down the equation triangle n c v n = Number of moles C = Concentration (mol l-1) V = Volume (litres, if in cm3 divide by 1000) Step 2 Cover up the variable you have to calculate, in this example its c, and this will give the equation. C = n/V Step 3 Substitute the values given in the question into the equation. n= 2 v = 200/1000 = 0.2 C = 2/0.2 = 10 mol l-1 Some examples require both triangles to be used together. Calculate the concentration when 20g of NaOH is dissolved in 250cm3 of water. If we are given a mass in a question the first thing we should do is calculate the gfm and then use the mole triangle to calculate n. 1Na = 23 1O = 16 1H = 1 23 + 16+1 = 40 n = m/gfm m=20 gfm =40 n = 20/40 = 0.5 We can now use the second triangle to calculate the concentration C = n/v C = 0.5/ 0.25 C = 2 mol/l n= 0.5 v= 250/1000 = 0.25 Use the procedures shown to carry out the following calculation. Calculate the volume needed to produce a 2 mol/l solution from 18.25 g of sodium chloride. Self Evaluation And Target Sheet Having analysed and evaluated your performance in this unit record areas of strengths and areas for improvement. Through discussion with your teacher set targets to improve your performance. Strengths Areas for Improvement Targets Pupil Teacher






