Switch-a-Roo 1 (adapted from reading on p143) Changes are

Switch-a-Roo 1 (adapted from reading on p143)
Changes are constantly occurring. Scientists try to determine whether these changes are permanent. Can you think of a change that is not permanent? Consider water freezing; is this change permanent? Liquid water can freeze and ice can melt. Liquid water can evaporate and then condense back into a liquid state. These processes are examples of changes that are reversible.
What about chemical reactions? Can they be reversed? You saw evidence that this was possible in part 1 of this activity. When particles collide with enough energy, products are formed. The particles of the products also collide and can reverse the original Note: making products is called the forward reaction. When reactant concentrations increase, the rate of the forward reaction increases for a moment, resulting in more product.
There is a high concentration of reactants and almost no products at the beginning of a chemical reaction. The forward reaction, which makes products, happens quickly because there are so many reactant molecules to collide with each other. The reverse reaction, which makes reactants, is slow because there are so few product molecules to collide with each other to make reactants. As the reaction happens the concentration of both the reactants and the products changes; thus, the rates of the forward and reverse reactions change. Eventually, the rate of the forward reaction equals the rate of the reverse reaction. The system is said to be at equilibrium. A reversible chemical reaction is in
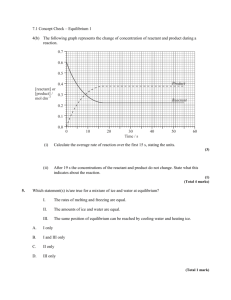
chemical equilibrium when the rate of its forward reaction equals the rate of its reverse reaction. A graph showing the concentration of reactants and products would have flat lines when the reaction is at equilibrium (showing no changes in concentration during this time).
It may appear that a chemical reaction has stopped when it reaches equilibrium. That’s because the concentrations of the reactants and products are not changing. However, the reaction has not stopped. On the molecular level, particles are still moving, colliding, and interacting. The equilibrium of chemical reactions is dynamic. What does that mean? Consider two island cities connected by a bridge. Cars move continuously from one city to the other. At equilibrium, the rate of the cars going in one direction equals the rate of the cars going in the opposite direction. Cars are not stopped (static) but are constantly in motion (dynamic). The cars are also moving both ways at the same time. The number of cars in each city is not changing because the cars leaving each city are equal to the cars entering each city.
A chemical reaction reaches equilibrium when the rates of the forward and the reverse reactions are equal. At this point, the concentrations of the reactants and the products are constant. But this does not mean that the concentrations of the reactants and the products are equal, just that there is no change in the concentrations. Think about the analogy of the two cities. The number of cars in each city is not changing, but that does not necessarily mean the number of cars in each city is the same.
Let’s summarize conditions of chemical equilibrium:
• forward reaction moves at the same rate as reverse reaction.
• forward and reverse reactions happen at the same time.
• the concentrations of the reactants and products are not changing (that does not mean that they are equal to each other).
Here’s a specific example in the form of a chemical reaction between gases:
CO
(g)
+ H
2
O
(g)
CO
2(g)
+ H
2(g)
What happens when H
2
O (g) and CO (g) are mixed? (See graph) Notice that the concentrations of the reactants (CO and H
(though not equal).
2
O) and products (CO
2
and H
2
) change and eventually become constant
Reactions rarely reach equilibrium with an equal amount of reactants and products – usually one is favored. In a reaction in which the forward reaction is favored, the concentration of the products is higher at equilibrium than the concentration of the reactants.