Trait - Wiley
advertisement

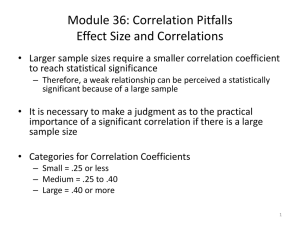
1 APPENDIX S1: Source of genotypes 2 Table A1 shows the source lake for each genotype. Clone lines were started from a 3 single parthenogenetic female from each lake, ensuring that individuals in each line 4 are genetically identical. 5 6 Table A1 Source lake characteristics for the four genotypes of Daphnia pulicaria. Location Surface Lake Clone (closest city) Lindsay 2C Total P (μg/L) 0.1250a 13.7a 8.0b 44°55’ N 76°40’ W 0.155b 26.0b Mississippi Station, ON 45°08’ N 78°30’ W Glen GLEN 0.1630d 15.0d Haliburton, ON 46°28’ N 80°56’ W Ramsey RAM 7.9520d 20.5d Sudbury, ON a Provided by Nelson Laboratory, Queen’s University, Kingston, ON Long 7 44°32’ N 76°23’ W Lake Opinicon, ON Area Maximum Depth (m) (km2) 4B 8 b From Reavie & Smol, 2001. 9 c From Reavie et al, 2006. 10 d Provided by Dorset Environmental Science Center (DESC), Dorset, ON 11 12 4.1c 10.3d 11.5d 13 APPENDIX S2: Maternal effects 14 Since we were interested in studying life-history traits across a range of resources that 15 went from near starvation to moderate abundance, there were some resource levels 16 where reproduction did not occur (see Results). As such, it was not possible to control 17 for maternal effects for all resource levels. To address the issue of maternal effects, 18 we conducted two rounds of experiments. In the first round, all offspring used in the 19 treatments came from mothers raised at the highest resource level and thus all life- 20 history responses include effects due to the difference between maternal and offspring 21 resource environment. In the second round, we took offspring from mothers raised at 22 resource levels where reproduction occurred and raised these individuals in the same 23 environment as their mother. Thus, we were able to compare the influence of maternal 24 environment on life-history trait correlations for the four highest resource levels. Note 25 that the two experiments were conducted concurrently, and are only distinguished for 26 ease of communicating the analyses and results. 27 To evaluate whether maternal effects had an influence on correlation 28 coefficient, we estimated correlation coefficients for those individual with maternal 29 effects, and those without maternal effects (see Methods), which yielded two 30 estimates of correlation coefficients for each genotype at the four highest resource 31 levels (0.5, 0.42, 0.34, 0.26 mgC/day). Reproduction was insufficient at lower 32 resource levels to raise enough individuals with controlled maternal effects. A two- 33 factor ANOVA with maternal effects and treatment indicated no significant impact of 34 maternal effects on correlation coefficient for any pair of traits (1- correlation: 35 F=2.9823, df=1,24, p=0.0970; 1- correlation: F=1.2463, df=1,24, p=0.2753, - 36 correlation: F=0.8018, df=1,24, p=0.3794). 37 38 2 39 APPENDIX S3: Life-history characterization 40 Figure S1 Example growth trajectories (circles) and corresponding fit von Bertalanffy 41 growth model (lines). Fit maximum growth rate (1) is shown beside each trajectory. 0.407 2.5 0.3882 0.36 0.3287 Length (mm) 2.0 0.2676 1.5 1.0 0 10 20 30 40 50 60 70 Age (days) 42 43 44 Figure C2 Correlation between maximum growth rate (1) and the common metric of 45 growth at fixed age (Vanni & Lampert 1992, Boersma & Vijverberg 1994), assuming 46 instar duration of 2.5 days (Taylor & Gabriel 1993). 3 0.45 Max Growth Rate 0.40 0.35 0.30 0.25 1.2 1.4 1.6 1.8 2.0 2.2 Length at age 9 47 48 49 50 51 52 4 53 APPENDIX S4: Shapiro-Wilk test for normality of life-history traits 54 Table D1 Results (p-value) of a Shapiro-Wilk test for normality of each life-history 55 trait (1=growth, =reproduction, =age at death). Values less than 0.05 indicate non- 56 normality. Genotype GLEN 4B Ramsey 2C Trait F=0.04 F=0.08 F=0.16 F=0.26 F=0.34 F=0.42 F=0.5 1 0.472 0.447 0.597 0.903 0.223 0.103 0.023 - - 0.048 0.029 0.100 0.218 0.157 0.000 0.036 0.520 0.011 0.000 0.159 0.027 1 0.092 0.048 0.105 0.034 0.453 0.489 0.373 - - 0.000 0.009 0.017 0.445 0.033 0.000 0.000 0.111 0.174 0.185 0.750 0.010 1 0.053 0.137 0.248 0.163 0.690 0.294 0.553 - - 0.000 0.077 0.026 0.138 0.328 0.000 0.001 0.628 0.082 0.024 0.002 0.000 1 0.017 0.495 0.004 0.046 0.661 0.307 0.212 - - 0.000 0.000 0.006 0.099 0.377 0.000 0.000 0.004 0.005 0.028 0.011 0.003 57 58 59 5 60 APPENDIX S5: Growth variation comparison 61 We observed growth variation among genetically identical individuals raised in 62 carefully controlled environments, which is common in zooplankton experiments (see 63 analysis in Ananthasubramaniam et al. 2011). To ensure this was not an artifact of our 64 transfer protocol, we compared the growth variance in our experiment to that found in 65 the literature. The highest resolved data is found in Lynch (1988), which shows an 66 increase in growth variance with incresing age that peaks around 10 days old (Figure 67 E1). Converting to a common basis of carbon per day, two of our food levels (0.5 and 68 0.42 mgC/day) correspond to the lowest food level studied in Lynch (1988). 69 Comparing these two studies, the dynamics of growth variance in our experiments 70 follow a very similar age pattern and is quantitatively similar in terms of both peak 71 and average variance values (Figure E2). 72 73 74 75 76 77 78 79 80 81 82 83 84 6 85 FIGURE E1. Dynamics of growth variance among genetically identical zooplankton 86 individuals from Lynch (1988) across nine food levels. 0.05 0.04 Variance 0.03 0.02 0.01 0.00 0 10 20 30 40 Age (days) 87 88 89 90 91 92 93 94 7 95 FIGURE E2. Dynamics of the growth variance among genetically identicals 96 observed in our experiments. a) Variance dynamics at 0.5 mgC/day for all four 97 genotypes (2C: blue, 4B: green, GLEN: yellow, RAMSEY: red) compared to the 0.1 98 mg C/day food level from Lynch (1988; dotted). b) Variance dynamics at 0.42 99 mgC/day for all four genotypes (2C: blue, 4B: green, GLEN: yellow, RAMSEY: red) 100 compared to the 0.8 mg C/day food level from Lynch (1988; dotted). 0.05 a 0.05 0.03 0.03 Variance 0.04 Variance 0.04 b 0.02 0.02 0.01 0.01 0.00 0.00 0 10 20 Age (days) 30 40 0 10 20 30 Age (days) 101 102 103 104 105 106 107 108 109 8 40 110 APPENDIX S6: Proportion reproducing 111 Figure F1. Proportion of adults that reproduced before death. Genotypes are shown in 112 different colors (red: RAMSEY, yellow: GLEN green: 4B, blue: 2C) 1.0 Proportion Reproducing 0.8 0.6 0.4 0.2 0.0 0.04 0.08 0.16 0.26 0.34 0.42 0.50 Treatment - mgC/vial/day 113 114 115 116 117 118 9 119 APPENDIX S6: Correlation coefficient analysis 120 Table G1 Statistical tests of whether the correlation coefficients differ from zero 121 (1=growth, 𝜔=reproduction, δ=age at death). Values in the top part of the table are p- 122 values from t-tests at each resource level. The next two blocks show if the null 123 hypothesis of no difference from zero is rejected (●) or not (◦). The first of these is 124 without adjusting the type I error rate, and the second is adjusting using Sidak’s 125 correction. Corresponding alpha values shown in parentheses in the first column. Trait Correlations F=0.04 F=0.08 F=0.16 F=0.26 F=0.34 F=0.42 F=0.5 1- - - 0.0084 0.0004 0.0077 0.0028 0.0377 1- 0.0122 0.0940 0.357 0.0031 0.0117 0.0017 0.0005 - - - 0.391 0.257 0.106 0.0221 0.0392 Trait Correlations Significance without adjusting type I error rate 1- (α=0.05) - - ● ● ● ● ● 1- (α=0.05) ● ◦ ◦ ● ● ● ● - (α=0.05) - - ◦ ◦ ◦ ● ● Trait Correlations Significance with adjusting type I error rate 1- (α=0.0102) - - ● ● ● ● ◦ 1- (α=0.0073) ◦ ◦ ◦ ● ◦ ● ● - (α=0.0102) - - ◦ ◦ ◦ ◦ ◦ 126 127 128 129 10 130 APPENDIX REFERENCES 131 Ananthasubramaniam, B., Nisbet, R.M., Nelson, W.A., McCauley, E. & Gurney, 132 W.S.C. (2011) Stochastic growth reduces population fluctuations in Daphnia–algal 133 systems. Ecology, 92, 362-372. 134 Boersma, M., & Vijverberg, J. (1994) Seasonal variations in the condition of two 135 Daphnia species and their hybrid in a eutrophic lake: evidence for food limitation. 136 Journal of Plankton Research, 16, 1793-1809. 137 Brookfield, J.F.Y. (1984) Measurement of the intraspecific variation in population 138 growth rate under controlled conditions in the clonal parthenogen, Daphnia magna. 139 Genetica, 63, 161-174. 140 De Graff, G. & and Prein, M. (2005) Fitting growth with the von Bertalanffy growth 141 function: a comparison of three approaches of multivariate analysis of fish growth in 142 aquaculture experiments. Aquaculture Research, 36, 100-109. 143 144 145 146 147 Ebert, D. (1994) A maturation size threshold and phenotypic plasticity of age and size at maturity in Daphnia magna. Oikos, 69, 309-317. Lynch, M. (1988) Path analysis of ontogenetic data. Size-Structured Populations (eds B. Ebenman & L. Persson), pp.29-46. Springer-Verlag, New York. Noonburg, E.G., Nisbet, R.M., McCauley, E., Gurney, W.S.C., Murdoch, W.W. & de 148 Roos, A.M. (1998) Experimental testing of dynamic energy budget models. 149 Functional Ecology, 12, 211-222. 150 Nisbet, R.M., McCauley, E., Gurney, W.S.C., Murdoch, W.W. & Wood, S.N. (2004) 151 Formulating and testing a partially specified dynamic energy budget model. 152 Ecology, 85, 3132-3129. 11 153 Reavie, E.D. & Smol, J.P. (2001) Diatom-environmental relationships in 64 alkaline 154 southeastern Ontario (Canada) lakes: a diatom-based model for water quality 155 reconstructions. Journal of Paleolimnology, 25, 25-42. 156 Reavie, E.D., Neill, K.E., Little, J.L. & Smol, J.P. (2006) Cultural eutrophication 157 trends in three southeastern Ontario lakes: a paleolimnological perspective. Lake and 158 Reservoir Management, 22, 44-58. 159 160 161 162 Taylor, B.E. & Gabriel, W. (1993) Optimal adult growth of Daphnia in a seasonal environment. Functional Ecology, 7, 513-521. Vanni, M.J. & Lampert, W. (1992) Food quality effect on life history traits and fitness in the generalist herbivore Daphnia. Oecologia, 92, 48-57. 12