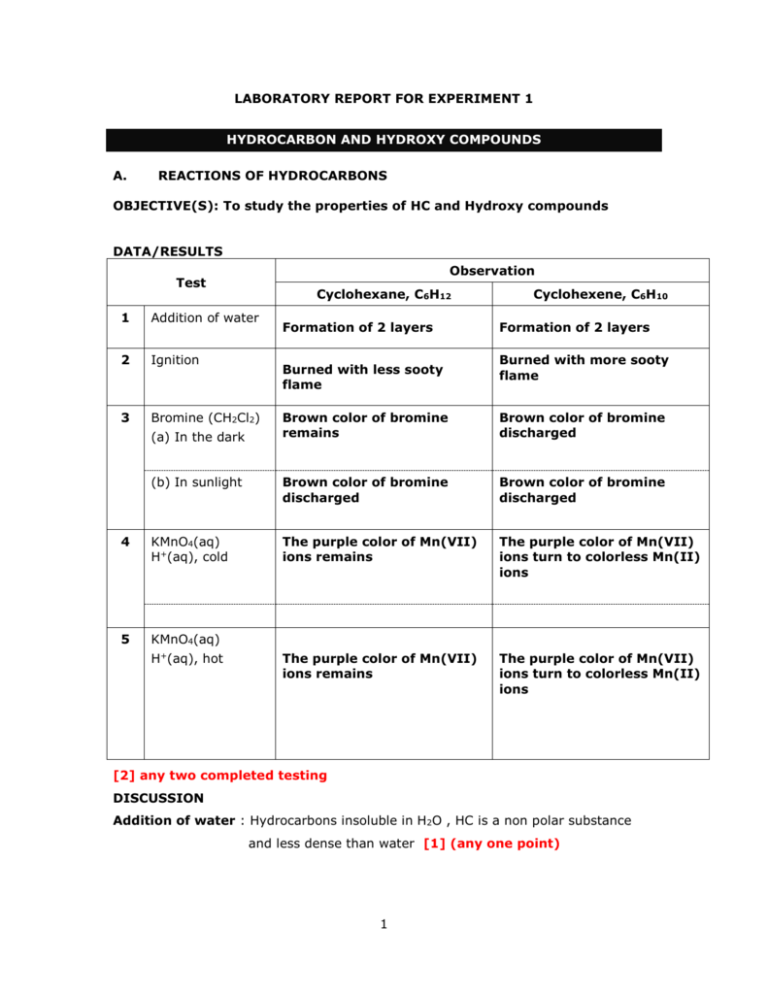
LABORATORY REPORT FOR EXPERIMENT 1
HYDROCARBON AND HYDROXY COMPOUNDS
A.
REACTIONS OF HYDROCARBONS
OBJECTIVE(S): To study the properties of HC and Hydroxy compounds
DATA/RESULTS
Observation
Test
Cyclohexane, C6H12
1
Addition of water
2
Ignition
3
Bromine (CH2Cl2)
Formation of 2 layers
Burned with less sooty
flame
Cyclohexene, C6H10
Formation of 2 layers
Burned with more sooty
flame
Brown color of bromine
remains
Brown color of bromine
discharged
(b) In sunlight
Brown color of bromine
discharged
Brown color of bromine
discharged
4
KMnO4(aq)
H+(aq), cold
The purple color of Mn(VII)
ions remains
The purple color of Mn(VII)
ions turn to colorless Mn(II)
ions
5
KMnO4(aq)
The purple color of Mn(VII)
ions remains
The purple color of Mn(VII)
ions turn to colorless Mn(II)
ions
(a) In the dark
H+(aq), hot
[2] any two completed testing
DISCUSSION
Addition of water : Hydrocarbons insoluble in H2O , HC is a non polar substance
and less dense than water [1] (any one point)
1
Ignition
: C6H12 + 9 O2 →
6 CO2 + 6 H2O
C6H10 + 17/2 O2 →
6 CO2 + 5 H2O more sooty flame
Bromination:
C6H12 + Br2 in CH2Cl2
uv
C6H10 + Br2 in CH2Cl2 →
C6H11Br + HBr
C6H10Br2
Oxidation:
C6H10 + MnO4-
cold
C6H10(OH)2 + Mn2+ @ MnO2 (brown ppt)
purple
C6H10 +
MnO4-
colorless
heat
HOOCCH2CH2CH2CH2COOH +
Purple
Mn2+
colorless
[1] on observation OR equation
2
B.
REACTIONS OF ALCOHOLS
OBJECTIVE(S):
DATA/RESULTS:
Observation
Test
1-propanol
2-methyl
2-butanol
-2-propanol
1
PCl5(s)
White fumes
produced
White fumes
produced
White fumes
produced
3
K2Cr2O7(aq)
The orange color
changes to
green/blue color
The orange color
changes to
green/blue color
The orange color
remains
4
Lucas test
No white ppt
formed at room
temperature
White ppt formed
after 5 minutes
White ppt formed
immediately
(10-30 mins)
(<5 mins)
No formation of
yellow ppt wt
antiseptic smell
yellow ppt wt
antiseptic smell
No formation of
yellow ppt wt
antiseptic smell
5
Iodoform test
[2] any two completed testings
DISCUSSION:
PCl5 :
CH3CH2CH2OH + PCl5 → CH3CH2CH2Cl + POCl3 + HCl (White fume)
CH3CH2CH(OH)CH3 + PCl5 → CH3CH2CH(Cl)CH3 + POCl3 + HCl (White fume)
CH3C(OH)(CH3)2
+ PCl5 → CH3C(Cl)(CH3)2 + POCl3 + HCl (White fume)
Oxidation, K2Cr2O7 (aq) :
CH3CH2CH2OH + Cr2O72- + 8H+ → CH3CH2COOH + 2Cr3+ + 7H2O
orange
green
CH3CH2CH(OH)CH3 + Cr2O72- + 8H+ → CH3CH2COCH3 + 2Cr3+ + 7H2O
orange
green
Lucas test:
3
CH3CH2CH(OH)CH3 + ZnCl2 + HCl → CH3CH2CH(Cl)CH3 + H2O + [Zn(OH)Cl2]
cloudy
CH3C(OH)(CH3)2 + ZnCl2 + HCl → CH3C(Cl)(CH3)2 + H2O + [Zn(OH)Cl2]
cloudy
rate of formation of cloudiness: 30 > 20 >10 alcohols
Iodoform test:
CH3CH2CH(OH)CH3 + I2 + NaOH → CH3CH2COO-Na+ + CHI3 + NaI + H2O
[1]
yellow ppt with antiseptic smell
CONCLUSION
[1] (any 1)
1. Cyclohexene is an unsaturated compound and is more reactive than cyclohexane.
2. Cyclohexene undergoes halogenation with or without uv light and can be
oxidized. Whereas cyclohexane only undergoes halogenation with the presence of
uv light and cannot be oxidized.
3. Confirmatory test for alcohol: PCl 5
4. Only 1o and 2o alcohol can be oxidized.
5. Iodoform test is used to test methyl alcohol.
REFLECTION
‘See you not that Allâh sends down water (rain) from the sky, and We produce
therewith fruits of varying colours, and among the mountains are streaks white and
red, of varying colours and (others) very black’ (35:27)
This verse shows the variety of Allah creation in this world. Each and every creation
has its own significance and play roles in the world.
REFERENCES
1.
Tan Yin Toon, Chemistry for Matriculation Semester 2, Oxford Fajar Sdn.Bhd.
(pg. 114-210)
2.
Holy Quran; Text and Translation by Abdullah Yusuf Ali (pg 426)
TOTAL MARKS 8
4
LABORATORY REPORT FOR EXPERIMENT 2
CARBONYL COMPOUND AND PHENOL
OBJECTIVE(S):To study the properties of carbonyl and phenol
A.
REACTIONS OF CARBONYL COMPOUNDS
DATA/RESULTS:
Test
Observation
Ethanal
Propanone
Benzaldehyde
1
Brady’s reagent
Yellow orange
precipitate is
formed
Yellow orange
precipitate is
formed
Yellow orange
precipitate is
formed
2
K2Cr2O7(aq)
Orange colour of
K2Cr2O7(aq) turns
green
Orange colour of
K2Cr2O7(aq)
remains
Orange colour of
K2Cr2O7(aq) turns
green
3
Tollen’s reagent
Silver mirror is
formed on the wall
of the test
tube/grayish black
precipitate
The solution
remains colourless
Silver mirror is
formed on the wall
of the test
tube/grayish black
precipitate
4
Fehling’s reagent
Blue colour
Blue colour
remains
Blue colour
remains
Yellow precipitate
with anticeptic
smell is formed
Colourless solution
changes to brick
red
5
Iodoform test
Yellow precipitate
with anticeptic
smell is formed
[any one correct testing and observations √1M]
5
DATA/RESULTS:
Observation
Test
Unknown
1
Brady’s reagent
Yellow orange precipitate is formed
2
K2Cr2O7(aq)
Orange colour remains
3
Tollen’s reagent
The solution remains colourless
4
Fehling’s reagent
Blue colour remains
5
Iodoform test
Yellow precipitate with antiseptic
smell is formed
Class/Family of unknown: Ketone @ aldehyde
[any one correct testing and observations √1M]
B.
REACTIONS OF PHENOL
DATA/RESULTS
Test
Observations
Phenol
1
Addition of
water
2
Acidity
Emulsion (cloudiness) is formed and colourless homogeneous
solution formed in hot water bath.
(a) Litmus
paper
(a)Blue litmus paper turns red
(b) (i) NaOH
(b)(i) homogeneous solution formed
(ii) HCl
(ii) emulsion formed
3
Iron(III)
chloride
Blue violet/purple solution formed
4
Bromine water
White precipitate formed/ brown colour of bromine discharged
[any one correct testing and observations √1M]
6
DISCUSSIONS:
Reaction of carbonyl compound
1.
Brady’s Reagent [√1M for equation OR observation]
a. Ethanal
O2 N
O
CH3 CH
+
O2 N
NH2 NH
NO2
CH3 CH
NNH
N2 O + H2 O
ethanal-2,4-dinitrophenylhydrazone
(yellow precipitate )
b. Propanone
O2 N
O
CH3 CCH3 +
O2 N
NO2
NH2 NH
CH3 C(CH3 )
NNH
N2 O
+ H O
2
propanone-2,4-dinitrophenylhydrazone
(yellow precipitate)
c. Benzaldehyde
O2 N
C6H5COH
+
O2 N
NO2
NH2 NH
C6H5CH
NNH
N2 O
+ H O
2
benzaldehyde-2,4-dinitrophenylhydrazone
(yellow precipitate)
2.
K2Cr2O7 [√1M for equation OR observation]
a. Ethanal
O
CH CH
3
O
K C r O /H +/w ar m
2 2 7
CH COH
3
ethanoic acid
+ Cr3+ (green/blue)
b. Benzaldehyde
O
O
CH
COH
+
K2 Cr2 O7 /H /warm
+ Cr3+ (green/blue)
benzoic acid
7
3.
Tollen’s reagent [√1M for equation OR observation]
a. Ethanal
O
O
CH3 CH
+
+
2 [Ag(NH3 )2 ]
+
-
-
3 OH
CH3 CO
2Ag(s)+ 4NH3
+
+
H2 O
Silver mirror
b. Benzaldehyde
O
O
CH
CO
-
+
+
2 [Ag(NH3 )2 ]
+
-
3 OH
+
2Ag(s)+ 4NH3
+
H2 O
Silver mirror
4.
Fehling’s reagent [√1M for equation OR observation]
~ positive for aliphatic aldehyde
a. Ethanal
O
O
CH3 CH
5.
+
2Cu2+
+
-
5OH
+ Cu2 O (s)
Brick red precipitate
-
CH3 CO
+
3 H2 O
Iodoform test [√1M for equation OR observation]
a. Ethanal
O
CH3 CH
O
+
3I 2
+
4
NaOH
CH3 CONa
+
3 NaI
+ H2 O
+ CHI3
Yellow precipitate with antiseptic smell
8
b. Propanone
O
O
CH3 CCH3 +
3I2
+
4
NaOH
CH3 CONa
+
3 NaI
+ H2 O
+ CHI3
Yellow precipitate with antiseptic smell
Reaction of Phenol
1.
Addition of water:
Phenol partially soluble in water. √1M
2.
Acidity
(a)
phenol is an acidic compound
⇌
C6H5OH + H2O
C6H5O- + H3O+
phenoxide ion
(b)
(i)
C6H5OH + NaOH
C6H5O- + Na+ + H2O
phenoxide ion(soluble)
(ii)
C6H5O-
+ H+
C6H5OH
cloudy as phenol precipitate out
3.
Iron (III) chloride
OH
OH
FeCl3
+ FeCl3 →
Blue violet soln
9
4.
Bromine in water [√1M for equation OR observation]
OH
OH
Br
Br
Br2 (aq)
Br
White ppt (2,4,6-tribromophenol)
CONCLUSION:
1. Carbonyl compounds undergo nucleophilic addition reaction
2. Aldehyde can be oxidized/reducing agent to carboxylic acid whereas ketones
cannot be oxidized
3. Ethanal and propanone can be distinguish from benzaldehyde by using
Iodoform test due to the carbonyl group being bonded to the methyl group
4. Phenol is an acidic compound which partially soluble in water.
REFLECTION:
‘He knows that which goes into the earth and that which comes forth from it, and
that which descend from the heaven and that which ascends to it. And He is the Most
Merciful, the Oft¬Forgiving ‘ (34:2)
Allah knows everything that happens around us, we as the caliph should observe the
change in the reaction to know actually happening.
REFERENCES:
1.
Tan Yin Toon, Chemistry for Matriculation Semester 2, Oxford Fajar Sdn.Bhd.
(pg. 219-250)
2.
Holy Quran; Text and Translation by Abdullah Yusuf Ali (pg 416)
TOTAL MARKS 10
10
LABORATORY REPORT FOR EXPERIMENT 3
HESS’S LAW - ADDITIVITY OF HEAT OF REACTION
OBJECTIVE
: To calculate the heat of reaction, H based on the Hess’s law
RESULTS
:
Experimental data
Reaction I
Reaction II
Reaction III
2.0000
2.0000
-
1
Mass of solid NaOH (g)
2
Volume NaOH (mL)
-
-
50.0
3
Volume water (mL)
100.0
-
-
4
Volume HCl (mL)
-
100.0
50.0
5
Total mass of solution (g)
102.0
102.0
150.0
6
Final temperature (C)
25.6
31.7
26.0
7
Initial temperature (C)
20.9
20.6
20.7
8
Change in temperature, T (C)
4.7
11.1
5.3
9
Heat, qreaction (kJ)
2.0
4.73
3.3
0.050000
0.050000
0.0500
-40
-94.6
-66
10
No. of moles of NaOH/mol
11
H (kJ/mol)
[√1M for H (kJ/mol) with –ve sign and correct arrangement (Rxn II>Rxn1 &RxnIII) ]
Answers to the questions
1. (a)
NaOH(s) + H+(aq) + Cl-(aq)
H2O(l) + Na+(aq) + Cl-(aq)
Na+(aq) + OH-(aq) + H+(aq) + Cl-(aq)
11
√1M
√1M
(b)
Heat of reaction I + heat of reaction III
= [-40 + (-72)] kJ/mol
= -106 kJ/mol
Heat of reaction II = -94.6 kJ/mol
2.
% Error = Theoretical H – Experimental H x 100%
Theoretical H
= (94.6 - 106 )kJ/mol 100%
94.6 kJ/mol
= 12 %
√1M
DISCUSSIONS:
Theory √1M
Heat, q is a transfer of energy between two objects at different temperature
Enthalpy change, H is the energy changes that occur in a chemical reaction
at constant pressure.
First law of thermodynamic:
- The law of conservation of energy
- Energy can be changed from one form into another
- It can neither be created nor destroyed
Hess’s law states that when reactants are converted to products, the change
in enthalpy is the same whether the reaction takes place in one step or in a
series of steps.
Hsoln is the enthalpy change when 1 mol of solute dissolves in a solvent.
Observations √1M
As the reaction occurs, the temperature of the solution increases/exothermic
rxn.
Sources of error √1M
Did not stir the solution simultaneously.
Did not add the NaOH(s) into the solution immediately.
Did not cover the styroform cup properly, thus some heat escape to the
surrounding.
12
Precautionary steps √1M
NaOH(s) is very hygroscopic (easily absorbed moisture from the air). Thus
weigh it and proceed to the next step without delay.
Constantly stir the solution.
The two solutions must be at the same temperature.
Cover the styroform cup properly to avoid the escape of heat to the
surrounding.
CONCLUSION:
The experimental value for the heat of reaction, H for the reaction between
NaOH(s) + HCl(aq) H2O(l) + NaCl(aq) is -92.8 kJ/mol
Based on the Hess’s law, the heat of reaction, H for the reaction between
NaOH(s) + HCl(aq) H2O(l) + NaCl(aq) is -85.1 kJ/mol
REFLECTION:
‘The Day that we roll up the heavens like a scroll rolled up for books(completed)even as We produced the first creation, so shall We produced a new one: a promise
We have undertaken; truly shall We fulfill it.’
(21:104)
This is about the Day of Judgment, where Allah promised how he created at the
beginning, He created the same for the last.
BIBLIOGRAPHY:
1. Brown, Lemay and Bursten; Chemistry: The Central Science, 10 th edition,
Pearson Prentice Hall, page 176-178
2. Holy Quran; Text and Translation by Abdullah Yusuf Ali, page 393.
TOTAL MARKS 8
13
LABORATORY REPORT FOR EXPERIMENT 4
DISTURBING THE POSITION OF EQUILIBRIUM – LE CHATELIER’S PRINCIPLE
OBJECTIVES
: √1M
-
To analyze the effect of concentration (common ion) and temperature on the
equilibrium position.
-
To explain the change of equilibrium position based on the Le Chatelier’s
principle
RESULTS
:
PART A: Effect of Temperature Change on a Physical System
Test conditions
Observations
Cooling to 0.0C
White crystals formed √1M
Warming to room temperature
White crystals dissolved
PART B: Common Ion Effect on a Chemical System
Test
tube
Conditions
Observations
1
Equilibrium without stress
Orange
2
Increase H+ added from H2SO4
Darker orange
3
Decrease H+ from added NaOH
Yellow √1M
4
After addition of H2SO4 to solution that
was added NaOH previously
14
Darker orange
PART C: Common Ion Effect on a Chemical System
Test
tube
Ions added
Observations
1
Control
Orange
2
Fe3+
Darker orange √1M
3
SCN-
Blood red
4
K+ and Cl-
5
OH-
Light yellow OR Light orange
6
Ag+
Pale yellow /cloudy
Pale yellow
OR Pale orange
DISCUSSIONS:
Theory
Equilibrium occurs when the concentrations of reactants and products remain
constant.
Equilibrium is established when the rate of forward reaction equals the rate of
backward reaction.
Factors that affect equilibrium:
- Concentration
- Temperature
- Pressure
- Catalyst
Le Chatelier’s principle states that if a system at equilibrium is subjected to a
change (stress), the equilibrium tends to shift so as to minimize the effect of
change (relieve the stress).
Observations and explanations
PART A √1M
The equilibrium as written is endothermic in the forward direction. When the
temperature is decreased, white crystal formed. This shows that the
equilibrium has shifted to the left (in the exothermic direction), therefore
releasing heat. When the temperature is increased, white crystal dissolves as
the equilibrium shifts to the right.
PART B√1M
Adding more acid will increase the concentration of H +(aq) ions. The
equilibrium position will shift to the right where some of the extra H + ions
added react with the CrO42- ions to form orange Cr2O72- ions and so minimize
the increased in the concentration of H+(aq).
15
Adding base (containing OH- ions) will reduce the H+(aq) concentration by
neutralization:
H+(aq) + OH-(aq) H2O(l)
The equilibrium position will shift to the left to minimize the decrease in
H+(aq) ion concentration, forming yellow CrO42- ions.
PART C√2M
Adding more FeCl3 will increase the concentration of Fe3+(aq) ions. As a
response, the equilibrium position will shift to the right where some of the
extra Fe3+ ions added react with the SCN- ions to form blood red [Fe(SCN)]2+
ions and so minimize the increased in the concentration of Fe 3+(aq).
Adding more KSCN will increase the concentration of SCN -(aq) ions. As a
response, the equilibrium position will shift to the right where some of the
extra SCN- ions added react with the Fe3+ ions to form blood red [Fe(SCN)]2+
ions and so minimize the increased in the concentration of SCN-(aq).
Adding KCl (containing Cl - ions) will reduce the Fe3+(aq) concentration by a
reaction:
Cl-(aq) + Fe3+(aq) FeCl3(s)
The equilibrium position will shift to the left to increase the Fe3+(aq) ion
concentration again, forming pale yellow solution.
Adding NaOH (containing OH- ions) will reduce the Fe3+(aq) concentration by
a reaction:
Fe3+(aq) + OH-(aq) Fe(OH)3(s)
The equilibrium position will shift to the left to minimize the decrease in
Fe3+(aq) ion concentration, forming yellow solution.
Adding AgNO3 (containing Ag+ ions) will reduce the SCN-(aq) concentration by
a reaction:
Ag+(aq) + SCN-(aq) AgSCN(s)
The equilibrium position will shift to the left to minimize the decrease in SCN(aq) ion concentration, forming yellow solution.
Sources of error
Do not wait until the brown precipitate of Fe(OH) 3 and white solid of AgSCN
to settle down , thus difficult to identify the color the resulting solution.
Use the same dropper for different solution may cause contamination.
Precautionary steps
Wait until the brown precipitate of Fe(OH) 3 and white solid of AgSCN to settle
down to see the color of the solution clearly.
16
CONCLUSION :
An increased in temperature shifts equilibrium in the endothermic direction; a
@decrease in temperature shifts equilibrium in the exothermic direction.
Equilibrium will shift to the right when the concentration of a reactant is
increased or the concentration of the product is decreased.
@
Equilibrium will shift to the left when the concentration of a product is
increased or the concentration of the reactant is decreased.
REFLECTION:
‘Glory to Allah who created in pairs all things that the earth produces, as well as their
own (human) and things of which they have no knowledge’
(Yaasin:36)
This experiment is one proved that everything created is in pairs. Like this
experiment that observed the equilibrium system, forward and reverse reaction,
there would be shift to the left or right in order to achieve a new equilibrium.
BIBLIOGRAPHY:
1. Chemistry, Principle and Reactions, Masterton and Hurley, 5th edition, page
335.
2. Brown, Le May and Bursten; Chemistry: The Central Science, 10 th edition,
Pearson Prentice Hall, page 649-655.
TOTAL MARKS 8
17
LABORATORY REPORT FOR EXPERIMENT 5
ACID-BASE TITRATION
OBJECTIVES:
To determine the molarity of acetic acid used
To obtain the shape the titration curve using interface
To determine the pH of solution at equivalence point through the graph
RESULTS:
Molarity of NaOH used/M
0.100 M
Volume of NaOH used/mL
(At equivalence point)
25.60 mL
Volume of CH3CO2H used/mL
25.0 mL
Table 1: Before equivalence point
Volume of
0.00
2.00
4.00
6.00
NaOH
added/mL
pH of
3.9
4.4
4.7
4.9
solution
Volume of
added
NaOH
pH of
solution
10.00
12.00
14.00
16.00
5.1
5.3
5.4
5.5
5.7
18.00 mL
20.00 mL
22.00 mL
23.00 mL
6.0
6.2
6.3
6.4
Volume of
2 drops
2 drops
2 drops
NaOH
(23.50
(23.60
(23.70
added
mL)
mL)
mL)
pH of
6.9
7.0
7.1
solution
√1M pH value at equivalence point
2 drops
(23.10
mL)
6.5
2 drops
(23.20
mL)
6.6
2 drops
(23.30
mL)
6.7
2 drops
(23.40
mL)
6.8
At end point
(permanent pale pink)
Volume = ___25.60_ mL
9.1
Table 2: After equivalence point
Volume of
2.00 mL 4.00 mL 6.00 mL 8.00 mL
NaOH
added
pH of
11.9
12.4
12.6
12.7
solution
Volume of
NaOH
added
pH of
solution
8.00
20.00 mL
13.1
18
10.00
mL
12.00
mL
14.00
mL
16.00
mL
18.00
mL
12.8
12.9
12.9
13.0
13.0
CALCULATIONS:
1.
Calculate the number of moles of NaOH from this experiment.
Moles NaOH =
(0.100 mol/L)(25.60 mL)(1 L/1000 mL)
=
2.56 × 10-3 mol
√1M
2.
Calculate the molarity of acetic acid used in this experiment.
Moles CH3COOH
=
(2.56 × 10-3 mol NaOH)(1 mol CH3COOH/ 1 mol NaOH)
=
2.56 × 10-3 mol
Molarity CH3COOH = (2.56 × 10-3 mol)(25.0 mL) (1 L/1000 mL)
= 0.102 M √1M
3.
Based on your graph, what is the pH of solution at equivalence point? Label it
on your graph.
√1M graph with correct shape
The pH of solution at equivalence point is 8.1
4.
Is the pH of the solution acidic, basic or neutral? Explain your answer briefly.
Basic
√1M
The acetate ion that is dissociated from the sodium acetate salt can
undergo hydrolysis in water to produce OH ion. √1M
DISCUSSIONS:
Theory
Titration curve is a plot of pH against volume of added base or added acid.
It shows the pH changes that occur when an aqueous acid reacts with an
aqueous base.
Phenophtalein is used as an indicator because its color changes around pH 810.
Observation √1M
As NaOH is added to the acid, a gradual increase in the pH is observed until
the solution gets close to the equivalence point.
Near the equivalence point, a rapid change in pH occurs.
Beyond the equivalent point, where more bases have been added than acid,
more gradual changes in pH are observed.
The end point is reached when the solution turns to permanent pale pink,
which is about 25.0 mL NaOH added.
The changes of pH around the equivalent point occur at pH 7-11.
Sources of error
Do not rinse the pH sensor with distilled water before and after measuring
each value of pH of solution.
Precautionary step
Rinse the pH sensor with distilled water before and after measuring each
value of pH of solution.
Constantly stir the solution.
19
CONCLUSION: √1M
The molarity of CH3COOH from the experiment is 0.102 M
REFLECTION
‘And He has cast in the earth anchorages (mountains standing firm) so that it should
not reel with you, and rivers and roads that possibly you would be guided’ (16:15)
This verse mentions many signs of creation that give evidence of the divinity of Allah
as reflected in the greatness of His creation. The Surah also mentions the many
blessings of Allah on His worshippers in His perfect knowledge, the greatness of His
wisdom and His precise planning.
REFERENCES/BIBLIOGRAPHY:
1. Silberberg, Chemistry: the Molecular Nature of Matter and Change, 5 th Edition,
page 841-850.
2. Holy Quran; Text and Translation by Abdullah Yusuf Ali, page 626.
TOTAL MARKS 8
20
LABORATORY REPORT FOR EXPERIMENT 6
REDUCTION POTENTIALS IN MICRO–VOLTAIC CELL
OBJECTIVES √1M
To measure the reduction potential of micro-voltaic cells
To rank metals according to measured reduction potentials
RESULTS
Data Table 1: Copper as the Reference Metal
No.
Combination
Potential (V)
Metal for Red (+) Tip
Metal for Black (-) Tip
1
Copper/Zinc
0.784
Copper
Zinc
2
Copper/Lead
0.201
Copper
Lead
3
Copper/Silver
0.796
Silver
Copper
4
Copper/Iron
0.507
Copper
Iron
√1M All values +ve
Data Table 2: Rank the Metals
Metal
Lowest (-) Reduction Potential, Eo (V)
Zinc
-0.784 V
Iron
-0.507 V
Lead
-0.201 V
Copper
0.000 V
Silver
+0.796 V
Highest (+) Reduction Potential, Eo (V)
√1M For arrangement of metals according to measured potentials
*For reference: Theoretical electrode reduction potentials Eo
Metal
Zinc
Iron
Lead
Copper
Silver
Eo (V)
- 0.762
- 0.409
- 0.127
+ 0.339
+ 0.799
21
Data Table 3: Predictions and Results
Half-Cell
Combination
Predicted Potentials (V)
Measured
Potential (V)
Percent Error
(%)
Zinc/Lead
-0.201 – (-0.784) = 0.583
0.576
1.20
Zinc/Silver
0.796 – (-0.784) = 1.580
1.523
3.61
Zinc/Iron
-0.507 – (-0.784) = 0.277
0.292
5.40
Lead/Silver
0.796 – (-0.201) = 0.997
0.938
5.92
Lead/Iron
-0.201 – (-0.507) = 0.306
0.315
2.94
Iron/Silver
0.796 – (-0.507) = 1.303
1.280
1.77
√1M
for
any
correct
calculation in predicted
potential columns
QUESTIONS
In a galvanic cell, what happen at the anode and cathode?
Anode: Oxidation
√1M
Cathode: Reduction √1M
What is the direction of electron flow? Explain.
Electrons flow out from the negative anode (electrons are released in the
oxidation process) through the external wire to the positive cathode (where
the electrons are accepted in the reduction process).
√1M
DISCUSSIONS
Theory
A voltaic cell uses a spontaneous oxidation-reduction reaction to produce
electrical energy. Placing a piece of metal into a solution containing a cation
of the metal produces half-cells.
In this micro-version of a voltaic cell, the half-cell is a small piece of metal
placed into three drops of corresponding cation solution on a piece of filter
paper.
A porous barrier or a salt bridge normally separates the two half-reactions.
Here, the salt bridge is made from several drops of aqueous sodium nitrate
(NaNO3) placed on the filter paper linking the two half-cells.
22
Observations
When the tip of the red(+) end of the voltage sensor is touched to one metal
and the tip of the black(-) end is touched to the other metal the positive
voltage reading is observed. By the way, the ends of the voltage sensor is
reversed if the voltage drops to negative value.
Sources of error
The piece of metal is not completely sand with sand paper.
The drops of each solution are not enough.
The addition of sodium nitrate solution is not enough.
Precautions √2M
Top side of the metals should be kept dry.
Carefully sand each piece of metal on both sides.
Dampen the filter paper with more NaNO3 solution from time to time during
the experiment.
The voltage reading must be positive as the redox reaction that occurs in the
voltaic cell is spontaneous.
CONCLUSION
The reduction potential sequence for the metals is:
Zinc, Iron, Lead, Copper, Silver.
REFLECTION √1M
‘For all there will be degrees (or ranks) according to what they did. And your Lord is
not unaware of what they do’ (6:132)
Allah has arranged and ranked everything in the world at their own place, shows that
Allah is very precise in everything (Al-Khabir-The Most Meticulous)
BIBLIOGRAPHY
1. Silberberg, Chemistry: the Molecular Nature of Matter and Change, 5 th Edition,
page 933-943.
2. Holy Quran; Text and Translation by Abdullah Yusuf Ali, page 130.
TOTAL MARKS 10
END
23









