File
advertisement

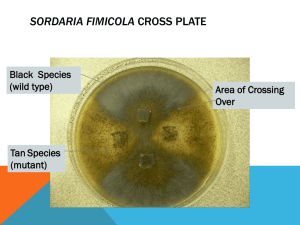
Lin 1 John Lin Biology 110H Section 2 Group: Jesse Weber and Marley Wolgast 11/14/13 George Inglis “Determining Baseline Asci Frequencies of Lab Strain Sordaria fimicola for Comparison to Strains Located at the Evolution Canyons” Lin 2 1. Introduction: Scientists want to know if environmental factors affect the types of offspring conceived through sexual reproduction. They know Meiosis produces gametes with various genotypic features. The process of recombination (or “crossing over”) where allelic pairs trade genetic information prior to Metaphase I is the biological process researchers are specifically looking at (Heredity and Life Cycle). It provides a source for genetic diversification. In other words, two homologous chromosomes may share genetic information for similar genes before separating in Metaphase I. If the environment does affect what kind of genes the organisms will express, then the genotypic changes should be revealed through the process of Meiosis. To study how the environmental conditions may affect gene expression, a research group from the Imperial College of Science, Technology and Medicine in England is studying a species of fungi, Sordaria fimicola, at a natural site that provides a territorial dichotomy. These sites are coined the Evolution Canyons and are located in Israel; they have a South Facing Slope (SFS) and North Facing Slope (NFS) where the former has higher temperatures and more arid while the latter is more lush, humid, and temperate (Singaravelan et al. 2010). S. fimicola lives in both regions and release either dark-colored asci (wild-type) or tan-colored asci. Scientists chose S. fimiola as their test subject because if its short life cycle, abundance, and production of patterned asci that depends on recombination (Hass and Ward 2010). Depending on what slope they live on, the environmental contrasts may selectively pressure them into expressing color pattern differences, providing evidence of changing crossover frequencies. (See Figure 1.) S. fimicola (a fungi) go through a life cycle called “Alternation of generations.” For these organisms, part of their life is spent as haploid (n) while the other part of their life is Lin 3 spent as diploid (2n) (Fungi I). Through Meiosis and followed by Mitosis, the fungi will form 8 asci that’s either dark or tan in the case of S. fimicola. Meiosis drives the sexual production part of the life cycle forming haploid cells from the original diploid cells. On the other hand, Mitosis guides cell replication and growth, so whatever ploidy an organisms begins with will be whatever it has after Mitosis. (See Figure 2.) Crossing over is another important event in the life cycle of fungi; it happens during Meiosis. During crossing over, two homologous chromosomes in a tetrad switch genetic information on parts of one of the non-sister chromatids (Hereditary and Life Cycle). The crossover frequency would be the amount of times crossing over occurs. After collecting the crossover frequency, map units can be calculated and they are the distance from the centromere to the end of the gene loci. It is measured by halving the crossover frequency. Overall, crossing over provides the various types of asci patterns found in S. fimicola. During the process of Meiosis and following Mitosis, S. fimicola produces three types of asci patterns—2:4:2, 2:2:2:2, and 4:4. The 2:4:2 and 2:2:2:2 patterns result from recombination while the 4:4 pattern come from no recombination. For the non-recombinant strain, a chromosome for dark asci pattern pairs with its tan homologous counterpart during Meiosis but does not share DNA. They then separate three times; twice during Meiosis and then once more during Mitosis. Because no recombination occurred, the dark-colored coding chromosome will form four dark asci while the tan-colored chromosome will also form four tan asci, ending with a 4:4 pattern. For the 2:2:2:2 asci pattern, recombination occurs between the two inner, non-sister chromatids. And for the 2:4:2 asci, recombination occurs in one of the inner chromatids with the homologous outer, non-sister chromatid. The recombinant strains will also follow three separations. (See Figure 3.) Lin 4 Overall, the purpose of the research wants to see if environmental cues has an effect on crossover frequencies, and the experiment done within the lab wants to find baseline crossover frequencies under optimal lab conditions. Researchers will find out if crossover occurs between the strands of S. fimicola and evidence of such crosses. To accomplish these tasks, scientists would have set up the two strains of S. fimicola in prepared agar cultures for mating. Then, they would examine the perithecia that includes asci under a microscope. Finally, data will be collected by counting the asci, giving results that will be used to calculate crossover frequency and map distance. 2. Materials and Methods (Hass and Ward 2010) The first step in the lab was to allow S. fimicola to mate. To do this, researchers obtained samples of dark-colored and tan-colored strains (grown on their own agar dishes). Using a newly-made, plain agar plate, the researchers divided the petri dish into four quadrants. Then, using a sterilized scalpel, they cut out two small square parts of agar from both color strains. Carefully, they would transfer the cut-out portions onto the fresh agar plate; the two dark-colored strains would be placed in quadrants diagonally opposite from each other and same goes for the tan-colored strains. Also, the perithecia-side of the cut-out portions were placed face down on the plate. Finally, the plate was sealed with tape and incubated at room temperature for two weeks. (See Figure 4.) After two weeks of incubation, the researchers reexamined their agar plate and should find newly formed perithecia bordering each quadrant. They would use these recent formation of perinthecia to examine for recombination under the microscope. First, they prepared a microscope slide by placing a drop of distilled water at the center of the slide. Using a sterilized inoculation loop, the researchers scraped off a small sample of perithecia from one Lin 5 of the bordering samples. Then, they would dip the loop into the drop of distilled water, trying to leave some of the sample in the liquid. The slides were finished with a coverslip that went over the water with the sample. Finally, before placing the slides under the microscope for viewing, the researchers have to gently tap the perinthecia under the coverslip (with the eraser on the pencil tip) to release the asci. Under the microscope, researchers would use 100x magnification to find the asci sample they want to observe and then score the asci under 400x magnification. (See Figure 5) The researchers looked for the three patterns (4:4, 2:2:2:2, 2:4:2) and tallied how many of each pattern they saw. From the scoring of the three patterns, the researchers would then calculate the crossover frequencies and map distances from their sample. The crossover frequency would equal the amount of recombinant crosses (2:2:2:2 and 2:4:2) divided by the total amount of crosses that constitute all three patterns. Then they multiplied the value by 100 to get a percentage. After finding the crossover frequency, to calculate the map distance, scientists halved the percentage number for the crossover frequency. 3. Results: Non-recombinant # of Type A Asci (4:4) 5 Recombinant # of Type B Asci (2:4:2) 2 Total # of Asci # of Type C Asci (2:2:2:2) 2 9 Total # of Recombinant Asci (B+C) 5 Table 1: Individual Data on Frequency of Recombinant and Non-Recombinant Asci of S. fimicola Non-recombinant # of Type A Asci (4:4) 11 Recombinant # of Type B Asci (2:4:2) 7 Total # of Asci # of Type C Asci (2:2:2:2) 8 26 Total # of Recombinant Asci (B+C) 15 Table 2: Lab Group Data on Frequency of Recombinant and Non-Recombinant Asci of S. fimicola Non-recombinant # of Type A Asci (4:4) 153 Recombinant # of Type B Asci (2:4:2) 122 Total # of Asci # of Type C Asci (2:2:2:2) 113 388 Table 3: Section Data on Frequency of Recombinant and Non-Recombinant Asci of S. fimicola Total # of Recombinant Asci (B+C) 235 Non-recombinant # of Type A Asci (4:4) 6,358 Recombinant # of Type B Asci (2:4:2) 5,105 Total # of Asci # of Type C Asci (2:2:2:2) 4,908 Total # of Recombinant Asci (B+C) 16,371 Lin 6 10,013 Table 4: Course Data on Frequency of Recombinant and Non-Recombinant Asci of S. fimicola NonRecombinant Recombinant # of Type A # of # of Type Asci (4:4) Type B C Asci Asci (2:2:2:2) (2:4:2) 153 122 113 Total # Of Asci Total # Recombinant Asci (B+C) Frequency of Recombinant Asci [(B+C)/ total # Asci] Frequency of Type B Asci (B/ total # Asci) 388 235 60.57% 31.44% Frequency Ratio of Type C B/C Asci (B/ total # Asci) 29.12% 1.08 Table 5: Section Data on Frequency of Recombinant and Non-Recombinant Asci of S. fimicola including calculations on crossover frequency and ratio of recombinant crosses NonRecombinant Recombinant # of Type A # of # of Asci (4:4) Type B Type C Asci Asci (2:4:2) (2:2:2:2) 6,358 5,105 4,908 Total # Of Asci 16,371 Total # Recombinant Asci (B+C) Frequency of Recombinant Asci [(B+C)/ total # Asci] Frequency of Type B Asci (B/ total # Asci) 10,013 61.16% 31.18% Frequency Ratio of Type C B/C Asci (B/ total # Asci) 29.98% Table 6: Course Data on Frequency of Recombinant and Non-Recombinant Asci of S. fimicola including calculations on crossover frequency and ratio of recombinant crosses Figure 1: Overhead and side images of Evolution Canyon depicting the SFS and NFS (Singaravelan et al. 2010) Figure 2: Alternation of Generation for the life cycle of Fungi (Cyr 2002) 1.04 Lin 7 Figure 4: Possible Asci patterns. First Diagram (top left) shows Meiotic and Mitotic separations that yield the nonrecombinant pattern (4:4). Second Diagram (top right) shows the 2:4:2 recombinant pattern.Third Diagram (bottom center)shows the 2:2:2:2 recombinant pattern. Figure 3: Placement of Wild-Type (darkcolored) and Tan-Type S. fimicola. Notice how Wild-Types are placed diagonally opposite from each other. Tan Types follow the same assortment. Figure 5: Sample of S. fimicola asci patterns under microscope. The asci are usually formed in patterns of 8 and researchers score only the 2:4:2, 2:2:2:2, and 4:4 patterns. (Singaravelan et al. 2010) From the results, the scientists noted some trends: recombinants occur more than nonrecombinants do on most levels of analysis. From the calculations above in Tables 1-5, the researchers have also noted the crossover frequency and map distance on the Section and Course level. The ratio of recombinants in Section and Course level data are about the same. Lin 8 4. Discussion Overall, recombination did occur between the two strains of S. fimicola in optimal lab setting because recombinant strains (2:2:2:2 and 2:4:2) were observed. In Tables 1-5, the researchers have calculated that crossover frequencies between the spore color gene and centromere to be 60.57% for Section level data and 61.16% on the Course level. From this, by halving the crossover frequencies, they found tthe map distance to be 30.29 mu (Section) and 30.58 mu (Course). From the two recombinant types of asci, the 2:4:2 pattern appeared more than the 2:2:2:2 pattern. But generally speaking, the numbers don’t differ by much because the ratio between the two types of asci came out to about 1. Thus, each type of pattern appeared at a frequency of about the same level. The trend observed from each Table reveals that recombination occurs more frequently compared to no recombination. This suggests that S. fimicola may also have cross over more in the wild in settings such as the Evolution Canyon. As more and more results came in, the researchers noted how the numbers became more uniform as well, providing more solid evidence that recombination occurs at about double the amount compared to nonrecombination. However, despite the evidence illustrated in Tables 1-5 that recombination did occur, the researchers also want to highlight that there may be sources of error that skew the actual results. First, there were errors in preparing the agar plates. While most groups succeeded in mating their wild-type and tan-colored S. fimicola, one group had contamination. When one group of researchers observed their agar plate, they noticed pink-colored mold or something else that had grown on their perithecium, rendering their sample inadequate for observation. Lin 9 Contamination could have occurred due to improper use of laboratory material. Through agar preparation step for mating S. fimicola, scalpels were instructed to be disinfected. The negligence of this step may have caused the contamination. Furthermore, not properly sealing the agar plate in the end could also make the culture of S. fimicola vulnerable to pollutants. Problems like contamination leads to other errors. Because some lab groups did not have samples of their own, they used other researcher’s samples. By double using samples, there may be bias in the results. Researchers may have used two samples that both contained a lot of recombinant asci; the true results could have been one sample with an abundant of recombinant asci while the other had little. However, because this kind of error has large effects on small samples of data, by amplifying the amount of asci scored to the Course level, the researchers could deter such errors. Another cause of double use could be the improper breakage of perinthecia to release the asci. By tapping too hard or too gentle, the asci may get destroyed or not enough asci were released. So multiple failures to properly release asci could cause researchers to use another lab group’s correctly microscope slides. From this experiment, researchers have prepared a sample baseline for S. fimicola in optimal lab setting. This means that if no environmental effects are at work, then S. fimicola would cross over at the frequency of around 60%. For the other group of researchers who are experimenting at the Evolution Canyons, they can use this lab baseline to compare with how S. fimicola mate in the wild. Their lab setup would be similar but their results may differ in that more crossing over would occur in SFS because of harsher conditions, which shows that environmental differences may affect the frequency of crossing over. Lin 10 In addition, for future experiments, scientists can conduct other studies at the Evolution Canyon. Currently, studies are done with how Spiny Mice express different fur colors depending on the slope they live on at Evolution Canyon (Singaravelan et al. 2010). Also, another research is done on Saccharomyces cerevisiae (a species of yeast) to see how the harsh conditions from over exposure from UV light may damage their DNA (Lidzbarsky et al. 2009). Both of these provide further insight on how environmental factors may affect how organisms express their genes. Lin 11 Reference Cyr, R. 2002. Fungi I - Evolution and Diversity, Phyla Chytridiomycota and Zygomycota. In, Biology 110: Basic Concepts and Biodiversity Course Website. Department of Biology, The Pennsylvania State University. http://www.bio.psu.edu/ Cyr, R. 2002. Heredity and Life Cycle. In, Biology 110: Basic Concepts and Biodiversity Course Website. Department of Biology, The Pennsylvania State University. http://www.bio.psu.edu/ Lidzbarsky GA, Shkolnik T, Nevo E (2009) Adaptive Response to DNA-Damaging Agents in Natural Saccharomyces cerevisiae Populations from ‘‘Evolution Canyon’’, Mt. Carmel, Israel. PLoS ONE 4(6): e5914. doi:10.1371/journal.pone.0005914 Meiosis and Genetic Diversity in the Model Organism, Sordaria. Written by Hass, C. and Ward, A. 2010. Department of Biology, The Pennsylvania State University, University Park, PA. Singaravelan, N., Pavlicek, T., Beharav, A., Wakamatsu, K., Ito, S., & Nevo, E. (2010). Spiny mice modulate eumelanin to pheomelanin ratio to achieve cryptic coloration in "evolution canyon," israel. PLoS One, 5(1) doi:http://dx.doi.org/10.1371/journal.pone.0008708