Lab Report - Personal.psu.edu
advertisement

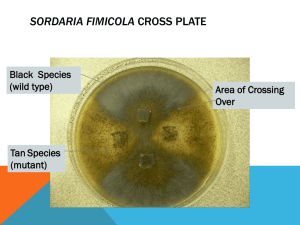
Gretchen Gurganus Bio 110 Lab Section Bradley Carlson October 19, 11 Anomalous Combinations of Asci in Sordaria fimicola due to Meiosis Introduction Sordaria fimicola is a fungi that is a part of the Ascomycota phylum and is an excellent example of how variations are produced in genetics. Organisms in this phylum have a defining reproductive characteristic called an ascus. The ascus, contained in the perithecium, is a sac-like cell containing 4 to 8 haploid spores. These spores are produced through the process of meiosis to make 4 haploid spores. They then undergo mitosis to make a complete 8 spore asci. When these spores are ready for dispersal, or when the perithecium is ruptured, the asci (multiple ascus) are dispersed. The process of meiosis and crossing over is displayed in the pattern of the spores in the asci. In the asci strains in S.fimicola, there are two different colors of spores. “When scientists first crossed a wild type strain of S.fimicola with a black spore coat with another strain with a tan spore coat they expected to find asci containing 8 spores in just two combinations. They discovered, in addition four, anomalous combinations” (Hass and Ward 2010). The expected combinations were WWWWTTTT and TTTTWWWW, while the anomalous combinations were WWTTWWTT, TTWWTTWW, TTWWWWTT, and WWTTTTWW (See Figure 1). These spores showed different combinations at different frequencies. To determine why these spores were in anomalous combinations and how often each of these combinations occurred, we observed multiple asci that were crossed with wild and tan strains and recorded the frequencies of each combination. Our class then predicted that pre-mitotic mixing caused the anomalous combinations and each combination had equal frequencies in this mixing. We also predicted that crossing over in the prophase stage of meiosis 1 caused the combination of 4:4 to be more frequent, while the 2:2:2:2 combination and the 2:4:2 combination have equal frequencies. We began this lab by splitting up a culture plate in four sections and making a cross between tan and wild Sordaria. After the fungi had crossed (approx. 2 weeks), we used an inoculating loop to scoop samples from the spots where tan and wild Sordaria had crossed and put them on a microscope slide lubricated with a drop of water. After pressing down the slide to release the asci, we observed multiple asci coming out of a single perithecium through a microscope. Making sure each asci had both tan and black spores, we counted the frequency of each 2:2:2:2, 2:4:2, and 4:4 combination and recorded the data. Results After finding a group of asci, we used 400x magnification to discover the types of combinations in each ascus. As you can see from Figure 2, the individual data contained 9 asci with the 4:4 combination, 5 asci with the 2:2:2:2 combination, and 6 asci with the 2:4:2 combination. The frequency ratio of anomalous asci out of the total number of asci is 11:20. Our group data (Figure 3) has 28 asci with the 4:4 combination, 14 asci with the 2:2:2:2 combination, and 22 asci with the 2:4:2 combination. Our frequency ratio was 36:64. As a whole class (Figure 4), we have 188 asci with the 4:4 combination, 120 asci with the 2:2:2:2 combination, and 139 asci with the 2:4:2 combination. Our class frequency is 259:447. Our class mapping distance was 28.9%. Discussion According to our findings as a class, 42% of asci were 4:4 combinations, 27% were 2:2:2:2 combinations, and 29% were 2:4:2 combinations. As a result, 4:4 combinations were much more common than 2:2:2:2 combinations and 2:4:2 combinations. The 2:2:2:2 and 2:4:2 combination had about equal frequencies. This data supported our class hypothesis of how anomalous combinations were formed by crossing over. For instance, we predicted that the frequency of 4:4 combinations would have occurred more than the other two, and that the other two would have had equal frequencies. Our results reinforced our hypothesis by showing matches in our predictions of frequencies. Our results also contradicted our other hypothesis of pre-mitotic mixing. In this hypothesis, we predicted equal frequencies in all of the combinations. This wasn’t true because 4:4 combinations occurred about 12% more frequently than the 2:2:2:2 and 2:4:2 combinations. The map distance of 28.9% also supported our hypothesis of crossing over. 28.9% describes the percentage recombination between two genes in correspondence with the centromere. “Frequency of crossover can be affected by location (crossing over is repressed close to the centromere) and proximity to another crossover” (“Introduction to Linkage Mapping”). Our 28.9% map distance location shows frequency in crossing over. Even though we had substantial support for our hypothesis in this experiment, there are always sources of error. Error could have occurred while the smaller groups were compiling data into a whole class result. There could have been miscalculations between the smaller groups that caused incorrect information for our data as a class. Also, some groups may have been making faulty observations while looking at asci because the tan and black spores looked very similar in color. In conclusion, this experiment demonstrated how genetic variations occur in meiosis through crossing over. It is important as biologists to recognize what genetic recombination are caused by and how it affects the order of the spores. After performing this experiment, I have a much better understanding of the effect of crossing over in meiosis in genes. It has lead me to wonder if in the future we could manipulate the map distance of the centromere and control the crossing over frequency. If we could do this, could we make an infinite number of combinations through the process of meiosis? Overall, this experiment furthered understanding of the effect of crossing over in meiosis. Species need genetic variation to help them survive natural selection. “Therefore, genetic variation is "insurance" for organisms against changing conditions because it helps to insure that some of their offspring will survive if conditions change” (Cyr 2002). This process is essential to life on earth. Figures Figure 1 4:4 Combination(Expected) WWWWTTTT TTTTWWWW 2:2:2:2 Combination(Anomalous) WWTTWWTT TTWWTTWW 2:4:2 Combination(Anomalous) WWTTTTWW TTWWWWTT Figure 2 Individual Data # of 4:4 # of asci 2:2:2:2 asci # of 2:4:2 asci Total # of asci 9 6 20 Group Data # of 4:4 # of asci 2:2:2:2 asci # of 2:4:2 asci Total # of asci 28 22 64 5 Total # Frequency anomalous of asci anomalous patterns 11 11:20 Figure 3 Figure 4 Class Data 14 Total # Frequency anomalous of asci anomalous patterns 36 36:64 # of 4:4 asci # of 2:2:2:2 asci # of 2:4:2 asci Total # of asci 188 120 139 447 Total # Frequency anomalous of asci anomalous patterns 259 259:447 References: Cyr, R. 2002. Heredity and Life Cycles. In, Biology 110: Basic concepts and biodiversity course website. Department of Biology, The Pennsylvania State University. http://www.bio.psu.edu/ Fancher, Lynn 2000. “Introduction to Linkage Mapping.” Division of Natural Sciences, College of DuPage. http://www.cod.edu/people/faculty/fancher/genetics/LinkageMapping2.htm Hass, C and Ward, A 2010. Meiosis and Genetic Diversity in the Model Organism, Sordaria. Department of Biology, The Pennsylvania State University, University Park, PA.