Silver Nitrate Lab
advertisement
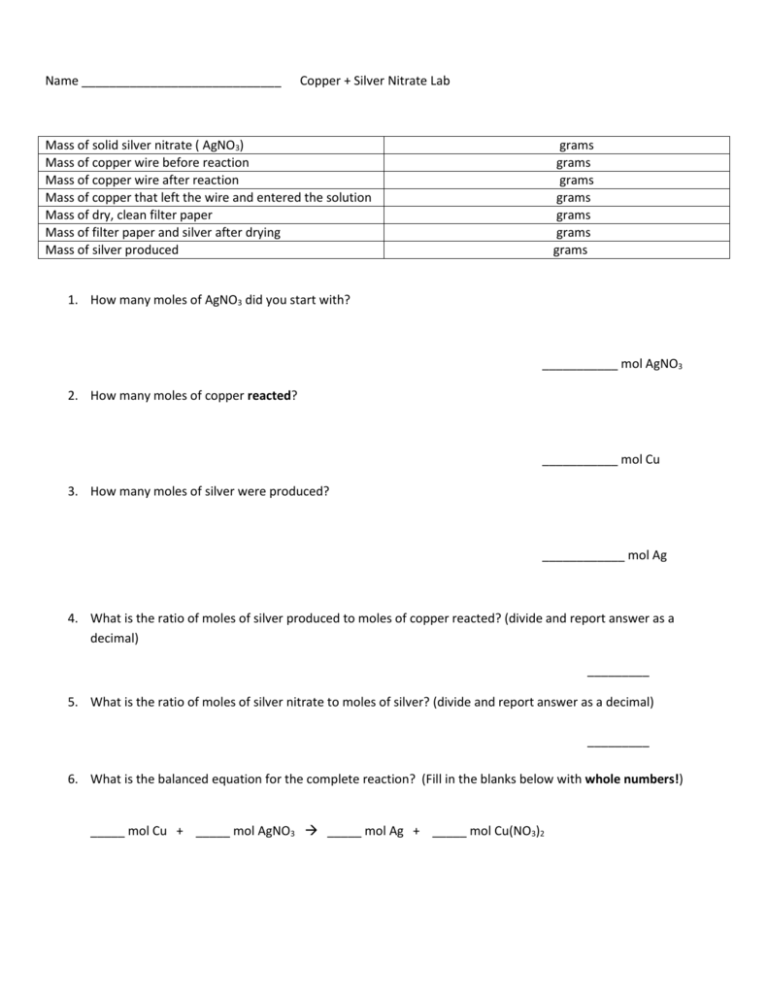
Name _____________________________ Copper + Silver Nitrate Lab Mass of solid silver nitrate ( AgNO3) Mass of copper wire before reaction Mass of copper wire after reaction Mass of copper that left the wire and entered the solution Mass of dry, clean filter paper Mass of filter paper and silver after drying Mass of silver produced grams grams grams grams grams grams grams 1. How many moles of AgNO3 did you start with? ___________ mol AgNO3 2. How many moles of copper reacted? ___________ mol Cu 3. How many moles of silver were produced? ____________ mol Ag 4. What is the ratio of moles of silver produced to moles of copper reacted? (divide and report answer as a decimal) _________ 5. What is the ratio of moles of silver nitrate to moles of silver? (divide and report answer as a decimal) _________ 6. What is the balanced equation for the complete reaction? (Fill in the blanks below with whole numbers!) _____ mol Cu + _____ mol AgNO3 _____ mol Ag + _____ mol Cu(NO3)2











