Cyanokit Administration Guidelines
advertisement

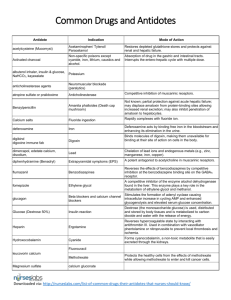
Guideline for the Administration of Cyanokit (Hydroxocobalamin) Hydroxocobalamin is the preferred treatment for symptomatic patients who either have a known or suspected exposure to cyanide or those that have been exposed to the products of combustion of an enclosed room fire in a structure. Patients exposed to the products of combustion will likely present with soot around the mouth or nose or in the larynx. Background: Cyanokit contains hydroxocobalamin, a form of vitamin B-12. It is used as an antidote to cyanide poisoning. Hydroxocobalamin works by helping cells in the body convert cyanide to a form that can be removed from the body through urination. Cyanokit is used in an emergency to treat cyanide poisoning. This type of poisoning can occur if you are exposed to smoke from a house or industrial fire, if you swallow or breathe in cyanide, or if you get cyanide on your skin. The presence and extent of cyanide poisoning are often initially unknown. There is no widely available, rapid, confirmatory cyanide blood test. Treatment decisions must be made on the basis of clinical history and signs and symptoms of cyanide exposure. If clinical suspicion of cyanide poisoning is high, Cyanokit should be administered without delay. Exposure to cyanide can mimic the same symptoms and presentation of hypoxia and/or CO exposure including respiratory or cardiac arrest. Again, it is important if the clinical suspicion of cyanide poisoning is high that a Cyanokit be administered without delay. Common signs and Symptoms of Cyanide Poisoning Symptoms Signs Altered Mental Status (e.g. confusion, disorientation) Seizures or Coma Headache Mydriasis Confusion Tachypnea / Hyperpnea (early) Dyspnea Bradypnea / Apnea (late) Chest tightness Nausea Hypertension (early) / Hypotension (late) Cardiovascular collapse Vomiting Plasma lactate concentration ≥8 mmol/L Paramedics in King County are authorized to administer Cyanokits in the following conditions: Symptomatic patients (altered mental status, hypotension, confusion) with known or suspected cyanide exposures with a GCS ≥ 8 and a plasma lactate measurement of ≥ 8 mmol/L. Any patient with a GCS < 8 (including cardiac or respiratory arrest) and a known or suspected cyanide exposure. Patients in cardiac or respiratory arrest should be administered the Cyanokit without delay. A blood sample should be drawn prior to administration, only if it can be done without delaying the administration of the kit and care of the patient. CYANOKIT is Pregnancy Category C and should be used during pregnancy only if the potential benefit justifies the potential risk. Safety and effectiveness of CYANOKIT have not been established in pediatric patients. All other normal ALS care and interventions must also be administered as indicated by the patient presentation. Drug Administration: The starting dose of hydroxocobalamin for adults is 5 g administered as an intravenous (IV) infusion over 15 minutes (approximately 15 mL/min). Depending upon the severity of the poisoning and the clinical response, a second dose of 5 g may be administered by IV infusion for a total dose of 10 g. The rate of infusion for the second dose may range from 15 minutes (for patients in extremis) to two hours, as clinically indicated. Physical incompatibility (particle formation) and chemical incompatibility were observed with the mixture of hydroxocobalamin in solution with selected drugs that are frequently used in resuscitation efforts. Hydroxocobalamin is to be administered through a dedicated peripheral IV following the package insert for reconstitution and administration. Instructions for Administration: 1. Each bottle needs to be reconstituted in 200ml of Normal Saline (Do not use Lactated Ringers or 5% Dextrose) 2. Reconstitute by placing the vial in upright position and adding 200 ml NS to the 200 ml line via the transfer spike provided. 3. Repeatedly invert each bottle for 60 seconds to mix. DO NOT SHAKE 4. Infuse over 15 minutes (approximately 15 ml/min.) 5. Depending upon severity of the poisoning and the clinical response, a second dose of 5g can be administered via IV infusion up to a total dose of 10g. (Infusion rate for second dose ranges from 15 minutes to 2 hours as indicated. 6. Any unused reconstituted Cyanokit should be discarded after 6 hrs . Adverse Reactions: Potential reactions to administration of Cyanokit are: Transient Chromaturia, Erythema, Rash, Hypertension, Headache, and inflammation at the injection site.