pbi12480-sup-0001
advertisement

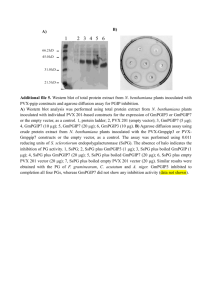
Supplemental data Methods Plant material and growth condition Nicotiana benthamiana plants were grown in soil at 26°C with a 16 h light / 8 h dark photocycle. Overexpression of SlbZIP1 and SlbZIP2 in Nicotiana benthamiana ClaI-NotI fragments containing the coding regions of SlbZIP1 and SlbZIP2 were individually subcloned into the pSfinx vector (provided by Prof. David Baulcombe, University of Cambridge, Cambridge, UK) (Takken et al. 2000). A ClaI-NotI GFP fragment was separately cloned into the pSfinx vector and was used as a negative control. Recombinant plasmids were introduced into Agrobacterium tumefaciens strain GV3101 (provided by Prof. David Baulcombe). Each transformant was grown overnight in YPE medium containing 50 μl.ml-1 kanamycin. Cells were harvested by centrifugation (4,000 × g), resuspended at OD600 = 1.0 in a buffer containing 10 mM MES-KOH, pH 5.6, 10 mM MgCl2 and 100 μM acetosyringone, and infiltrated into fully expanded N. benthamiana leaves with a needleless syringe as described in Zhu et al. (2012). After 24 h incubation, leaf samples were collected, immediately frozen in liquid nitrogen, and stored at −80°C until required. RT-PCR analysis Total RNA samples were prepared from N. benthamiana plants using Sepasol-RNA I Super (Nacalai Tesque, Kyoto, Japan). RNA was reverse-transcribed as described previously (Zhu et al. 2012) and the resultant cDNA was used for PCR analysis with the primers listed in Table S1. Table S1. Primers used in this study (continued) (continued). Fig. S1. SlbZIP1 and SlbZIP2 transcriptionally activate the expression of ProDH and ASN. The coding regions of SlbZIP1 and SlbZIP2 were separately cloned into the pGR106 vector (provided by Dr. David Baulcombe). The GFP fragment was also cloned into the pGR106 vector as a control. Plasmids were introduced into Agrobacterium tumefaciens GV3101 and the bacterial cultures used to infiltrate Nicotiana benthamiana leaves. Leaf discs (12 mm diameter) were removed using a cork-borer 24 h after infiltration. Total RNA was extracted from leaf discs and was subjected to RT-PCR analysis. ProDH, proline dehydrogenase gene; ASN, asparagine synthase gene. EF1-α was used as a loading control. Cycle numbers of PCR were displayed in right margin. References Takken FLW, Luderer R, Gabriels SJEJ, Westerink N, Lu R, de Wit PJGM, Joosten MHAJ (2000) A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J 24:275–283. Zhu XJ, Thalor SK, Takahashi Y, Berberich T, Kusano T (2012) An inhibitory effect of the sequence-conserved upstream open reading frame on the translation of the main open-reading frame of HsfB1 transcripts in Arabidopsis. Plant Cell Environm 35:2014-2030.