Lab #14 Solubility & Forces - NGHS
advertisement

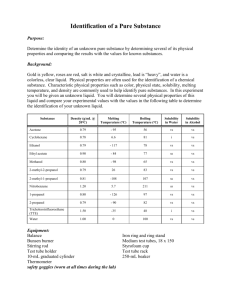
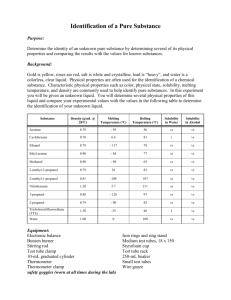
Lab #14: Liquids & Solubility: Cool Solubility Reactions Prelab Questions: How can VSEPR models help predict intermolecular bonding? Answer these pre-lab questions BEFORE beginning the experiment. 1. Predict whether the following molecules are polar or nonpolar. Justify your answer using VSEPR models. Draw them and fully explain your reasoning! a) oxygen difluoride, OF2 b) methane, CH4 c) carbon disulfide, CS2 d) fluoromethane, CH3F e) hydrogen peroxide, H2O2 f) ammonia, NH3 2. As noted by your teacher a couple of minutes ago, the weakest attraction between molecules are collectively called Van der Waals forces. For each of the above substances, list the kinds of attractive forces between molecules that are expected. Molecule LDF Dipole-dipole H-Bonds Oxygen difluoride methane Carbon disulfide Fluoromethane Hydrogen peroxide Ammonia 3. What two conditions are necessary for molecules to be polar? 4. If water had a linear molecular shape, would the molecule be polar or nonpolar? Explain your answer. 5. When will hydrogen bonding occur? Give an example of a liquid other than water, in which this type of force is important. Part 1: Crystals In and Out Problem: How does solubility change with temperature? Materials: Stirring rod Thermometer Large test tube Metric balance Potassium dichromate crystals (K2Cr2O7) Bunsen burner Ring stand Hot water bath Cold water bath 10 ml graduated cylinder Procedure: 1. Record a hypothesis to answer the problem. 2. Prepare a hot water bath by using a 400 ml beaker about 2/3 full of water. Place it on the hot plate. 3. While the water is heating, add between 4 and 5 grams of K 2Cr2O7 to a large test tube. Record the exact mass of the K2Cr2O7 in the data table below. 4. Measure out exactly 7.0 ml of water and pour it into the test tube. 5. Immerse the test tube in the hot water bath. 6. Insert a thermometer into the test tube and carefully stir with a stirring rod, not the thermometer, until the crystal has completely dissolved. This will not happen until the mixture becomes relatively hot. 7. Once the solid has dissolved, remove the test tube from the hot water bath and place it in a test tube rack. Stir gently as the solution cools. 8. When the first trace of crystals appear in the test tube, the solution is saturated. Record the temperature at which this occurs. Save this solution. 9. Pour exactly 3.0 ml of water into the test tube. The total volume of the solution in the test tube should now be 10 ml. 10. Lower the test tube into the hot water bath and stir until the salt crystals are again completely dissolved in the solution. 11. Remove the test tube from the hot water bath, stir again, and record the temperature at which the first crystals appear. 12. Repeat steps 8-10 four more times, adding 3.0 ml of water each time until a total of 22.0 ml of water have been added. Remember to record the crystallization temperatures in the data table. 13. When you are finished, give the test tube to your teacher for disposal. Clean up your lab area. Data: Mass of the K2Cr2O7 = Total Volume of Solution 7 ml 10 ml 13 ml 16 ml 19 ml 22 ml Crystallization Temperature Solubility (g/100 ml H2O) Questions: 1. Calculate the solubility of K2Cr2O7 at each of the 6 experimental temperatures. Assume 1 ml of water has a mass of 1 gram. Record the calculated solubilities in the data table above. 2. On a sheet of graph paper, prepare a plot of the solubility of K 2Cr2O7 versus the temperature. Be sure to place the dependant and independent variables on the appropriate axes. Include a title on your graph. 3. What does your graph indicate about the relationship between solubility and temperature? 4. Look up the solubility of K2Cr2O7 at various temperatures in the Handbook of Chemistry and Physics. Enter these accepted values on your solubility graph and draw a curve connecting the values using a different color pen. Be sure to includes a key on your graph indicating which line represents your experimental data and which is the plot of the actual curves. 5. How does the accepted solubility curve compare to your experimental solubility curve? 6. Name several sources of error that might explain any differences in the curves. Part 2: Yellow Trouble Problem: How do the concentrations of the reactants affect the amount of precipitate formed in a reaction? Materials: Potassium iodide crystals (KI) Lead nitrate crystals (Pb(NO2)2) 25 ml graduated cylinders 10 ml graduated cylinders Stirring rod Metric balance 4 test tubes Test tube rack Introduction: It’s time to put your new solution calculation skills to the test. When a chemical solution is prepared by your teacher, the proper calculations must first be completed. In this exploration experiment, you will be taking on the job of the teacher by determining the amounts of solid that must be weighed out to prepare different solutions. Hypothesis: Before actually performing the experiments, look closely at the ingredients for the four reactions. A solid, PbI2, will form when the 2 chemicals combine. Which reaction do you expect to produce the largest amount of solid? Explain your prediction. Procedure: 1. Do all the calculations necessary to determine the number of grams of each solute needed to prepare the solutions with their given concentrations. Show all of your calculations, including units. Solution 1: 20 ml of 0.2 M KI Solution 2: 20 ml of 0.2 M Pb(NO2)2 Solution 3: 20 ml of 0.4 M KI Solution 4: 20 ml of 0.4 M Pb(NO2)2 2. Once your teacher has checked your calculations, you may proceed to prepare the four solutions. Be extremely careful in weighing out your solids because you will be measuring fairly small amounts. You will be graded on the accuracy of your results. 3. Pour each of the solutions into four separate large test tubes and place them in a test tube rack. Label the solutions according to their concentrations and content. 4. Now, use these solutions to carry out the four chemical reactions outlined in the table below. Combine the proper portions of each chemical in a small graduated cylinder. Make sure to wash the cylinder between reactions. 5. Do not throw your precipitate down the drain or in the garbage. Your teacher will show you where to properly dispose of your chemicals. 6. Clean up your lab area. Data: Reaction Letter A B C D Amount of KI Amount of Pb(NO2)2 10 ml of 0.4 M KI 10 ml of 0.4 M KI 10 ml of 0.2 M KI 10 ml of 0.2 M KI 10 ml of 0.4 M Pb(NO2)2 10 ml of 0.2 M Pb(NO2)2 10 ml of 0.4 M Pb(NO2)2 10 ml of 0.2 M Pb(NO2)2 Height of the Precipitate (ml) Questions: 1. Write a balanced equation for the reaction that occurred above. Keep in mind that the solid formed is lead iodide. 2. Using the data above, determine the limiting reagents in each equation (if applicable), and the amounts of each product produced. 3. Which of the reactions formed the largest amount of precipitate? 4. Was the prediction you made correct? 5. Explain the similarities in the heights of the precipitates formed in reactions A and B. Part 3: A Salty Dilemma Problem: How can you determine the solubility of sodium chloride? Materials: Saturated salt solution Bunsen burner Watch glass Thermometer Ring stand Tongs Evaporating dish Procedure: 1. Record a hypothesis to answer the problem. 2. Measure out approximately 20 ml of saturated salt solution. 3. Take the temperature of the solution and record it in the data table below. 4. Weigh a dry, clean evaporating dish and a large watch glass and record it in the data table below. 5. Pour the measured salt solution into the evaporating dish. Cover it with the watch glass. 6. Weigh the dish, glass and solution. Record the weight in the data table below. 7. Place the evaporating dish on the ring stand. 8. Heat the solution gently, being careful not to let the solution boil too vigorously. If you allow your solution to boil over, you will need to start again. Low heat for about 10 minutes should be enough to evaporate all the liquid. 9. When evaporation is complete, allow the dish and glass to cool completely. 10. Weigh the dish, glass, and solid. Record the weight in the data table below. 11. Calculate the solubility of the salt from your data. Data: Temperature of the solution Mass of the evaporating dish and watch glass Volume of the liquid Mass of the dish, glass, and solution Mass of the dish, glass, and solid after evaporation Mass of the solid (salt) Solubility of NaCl (g/100 ml H2O) Questions: 1. What is the accepted value of solubility of NaCl in water? (Use the Handbook of Chemistry and Physics.) 2. How does your experimental solubility compare to the accepted value (is it too high or too low or about the same)? 3. Explain and differences you observed between your experimental and accepted solubility values. (What may have gone wrong?) Part 4: Soda Solution Problem: What is the concentration of carbon dioxide gas in soda? Materials: Soda Metric balance Graduated cylinders Beakers Hot Plate Introduction: Everyone has had soda. Have you ever given much thought to what is in it? Soda has a fairly simple composition: water, sugar, flavoring, and carbon dioxide are the main ingredients. Procedure: Record a hypothesis to answer the problem. Your challenge is to design an experiment that will allow you to determine the concentration of carbon dioxide in a sample of soda. Write your procedure below. Have your teacher check your idea before you begin collecting your data. Remember, your goal is to be able to calculate the concentration of CO 2 in your soda sample. Make certain you have collected the right kind of data to do this. Create a table for your data. Questions: 1. Calculate the molarity of CO2 in soda. Show all of your calculations. 2. Using your data, determine the mass of CO2 in a 2 L bottle of soda. Show your work. 3. Do you think your CO2 calculation is truly representative of the actual mass of CO 2 in the soda? Why or why not. 4. Check with another person in the lab who used a different kind of soda. Did you get the same results? Why or why not? Part 5: Milky Solutions Overview: Oil causes the food coloring to circulate in the milk making swirls of color Equipment: Milk (in a cup or single serving carton with the lid cut off) Food colors vegetable oil a table to place the milk on Procedure: 1. Put the milk in a cup, about 1/4 full. 2% or higher fat content works the best. 2. Place one drop of each color of food coloring in each corner of the carton or near the sides of the glass. 3. Place a drop of vegetable oil in the middle of the cup. Be careful not to move the glass or to shake the table. After a short time the colors swirl and mix, it looks really cool. 4. Try it with different fat percentages and see how it affects the patterns or the rate of swirling. 5. Record your observations below. Data and Analysis: Type of Milk Swirl Pattern Time to Swirl Skim Milk 1% Milk 2% Milk Whole Milk Questions: 1. Which milk took the longest time to swirl? The shortest? 2. What are the differences between the 4 types of milk? 3. Why does the food coloring swirl like this in milk? Use the terms miscible and immiscible in your explanation. 4. Do some research…is milk good for you or not? Support your answer. Part 6: Polarity & Solubility Molecules with polar bonds can be polar or non-polar. If they are completely symmetrical, the symmetry of the molecule can cancel out the polarity of the bonds. If a molecule is polar, it may have greater attraction for other polar molecules than non-polar molecules will. Non-polar molecules have an even distribution of charge, and will not attract one another, and thus will mix quite well. In general, the phrase “ Like dissolves like” describes the solubility of solutes in solvents. That is, polar solvents such as water, will easily dissolve other polar molecules; polar solvents will also dissolve ionic compounds because ionic compounds represent the extreme case of a polar molecule. Non-polar solvents will generally dissolve non-polar solutes. In this activity, you will determine the solubility of a variety of solutes in two different solvents, water and hexane. Procedure: 1. Obtain 7 test tubes of the same size, and place 5 mL of water in each one. (After you have measured the first one, you may estimate the 5 mL level in the other 6 tubes.) 2. Test the water solubility of each of the seven solutes by adding a different solute to each test tube. For liquid solutes, add about 20 drops. Transfer a pea-sized sample of each solid solute using a spatula or a wood splint. Gently mix the test tube and contents by flicking the bottom with your finger. 3. Record your observations in your lab book: S = soluble, SS = slightly soluble, IN= insoluble. 4. Discard the samples into the waste container labeled “Water Waste.” 5. Clean and dry the test tubes and repeat using hexane as the solvent. 6. Dispose of the hexane and solute wastes in the container labeled “Hexane Waste.” 7. Solute Water ( H2O) Hexane (C6H14) Urea ( CO(NH2)2 ) Iodine ( I2) Ammonium chloride Naphthalene ( C10H18) Copper(II) sulfate Ethanol ( C2H5OH) Sodium chloride Questions: 1. Which solutes were more soluble in water than in hexane? Why? 2. Which solutes were more soluble in hexane that in water? Why? 3. What can be said about the polarity of each of the solutes? Part 7: Capillary Action Capillary action depends partially on the polarity of the molecules of the liquid and of the material that forms the tube or space into which the liquid rises. A polar attraction between the material of the tube and the molecules of a polar liquid causes the liquid to rise into the tube. As one molecule moves up, it attracts neighboring molecules which, in turn attract their neighbors. Once the upward attractive force is equal to the downward force of gravity, the liquid stops rising. An extreme example of this phenomenon occurs in giant trees like redwoods. It is estimated that these large trees move about 2000 liters of water each day from their roots to the uppermost leaves by capillary action. Procedure: 1. Obtain a well-plate, eight capillary tubes, and a container of water. 2. Place a few drops of water in one of the wells in the plate. 3. With one end of the tube, touch the water. The top of the tube must be open. Leave the tip of the tube in contact with the liquid for at least 15 seconds. 4. Once the liquid stops rising, remove the tube from the well and measure the height of the column of liquid. Record the value. 5. Drain the capillary tube by touching its tip to a paper towel. 6. Repeat the procedure to allow liquid to rise into the tube 2 more times. Record the heights in the table. 7. Repeat the procedure using different liquids in the wells, and a new capillary tube for each liquid. (Note: the glycerol will take more than 15 seconds to reach its final height.) 8. Calculate the average height of the liquid columns for each substance tested. Liquid Water Ethanol Ethylene glycol Glycerol Mineral Oil Propanol Salt Water #1 ( Height) #2 #3 Average Based on the heights of the liquid columns, draw a conclusion about the relative polarity of the solvents tested. Part 8: Introduction to Intermolecular Forces in Solution Hydrogen bonding is due to the polarity of the oxygen-hydrogen, nitrogen-hydrogen, or fluorine-nitrogen bonds in molecules. Hydrogen bonding is an intra-molecular bond. That means it occurs between molecules, not within a molecule. Hydrogen bonding in water is responsible for some of water’s interesting properties. One of these properties is surface tension. In this activity, you will investigate hydrogen bonding and surface tension by seeing how many drops of a liquid you can place on a penny before it runs over. Trial number Number of drops Trial #1 Trial #2 Trial #3 average You will be given three solutions: sodium carbonate, sodium chloride and a 1% liquid detergent sample. Your task is to design and carry out an experiment to determine their effect on the surface tension of water. Design a procedure which includes a control, steps that will ensure that the results are reproducible, and predictions of the effect of each solute. Create a table that will organize your data clearly. Questions: 1. Compare the average number of drops placed on your penny with the results obtained by some of the other lab groups. What might account for differences? 2. What were your predictions for the effect of each solute on the surface tension of the water? 3. What was your control? 4. What steps did you include in your procedure to increase the reliability or reproducibility of your results? 5. Will the addition of salts always change the surface tension of water? Why or why not? 6. What effect did the detergent have on the surface tension? 7. What are some practical applications of what you tested and learned?