Supplementary Information
advertisement

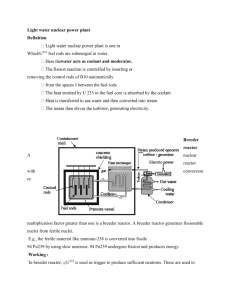
Supplementary Information Versatile, High Quality and Scalable Continuous Flow Production of Metal-Organic Frameworks Marta Rubio-Martinez,1 Michael P. Batten,*2 Anastasios Polyzos,3 Keri-Constanti Carey,1 James I. Mardel,3 Kok-Seng Lim1 and Matthew R. Hill*3 1 CSIRO Process Science and Engineering, Private Bag 33, Clayton South MDC VIC 3169 Australia . 2 CSIRO Earth Sciences and Resource Engineering Private Bag 33, Clayton South MDC VIC 3169 Australia. 3 CSIRO Materials Science and Engineering, Private Bag 33, Clayton South MDC VIC 3169 Australia . S1. General description of the continuous flow reactor process S1.a. Continuous Flow MOF Synthesis (Mesoscale) Experiments were performed using a commercially available continuous flow reactor Vapourtec R2+/R4 (www.vapourtec.co.uk) shown in Supplementary Figure 1. In a typical synthesis of metal-organic frameworks, separate solutions of the precursors are directed into the reactor by HPLC pumps through a T-type static mixer to promote complete mixing of the separate reagent streams. The combined mixed reactants are then directed into the heated reactor zone of the Vapourtec R4 unit which comprises coiled tubular reactors fabricated from perfluoroalkoxy polymer (PFA) tubing (internal diameter of 1mm and a volume of 10 mL for each tubular reactor). The reactor volume is readily increased by connecting the coiled reactor tubes in series (up to four coils for a single Vapourtec R4 unit). On exiting the reactor zone, the stream is passed through a back-pressure regulator (Upchurch) (100 psi) to maintain constant the pressure of the flow stream. The exiting product stream was then collected into a volumetric flask (100 mL) whereupon it was cooled to room temperature. Figure S1. Photograph of the Vapourtec R2+/R4 flow reactor consisting of two PFA polymer tubular reactors used in a typical mesoscale synthesis of MOFs. The system comprises the pumping and reagent selection module (R2+, top stage) and the four channel air-circulated heating reactor coils (R4; lower stage). S1.b. Continuous Flow MOF Synthesis (Macroscale) Continuous flow scale-up synthesis of were performed on a Salamander Flow Reactor (Cambridge Reactor Design Ltd., Cottenham, UK) as previously detailed by Micic et. al. [1]. Briefly, comprises a serpentine stainless steel tube (8 mm o.d., 6 mm i.d., 108 mL volume) and a thermostatically controlled electrical heating system (ambient to 150 °C). An inline back-pressure regulator, situated at the outlet of the reactor, allows for fine tuning of the reactor pressure (up to 20 bar). Static mixer units are placed within the linear sections of the reactor tubing to promote tubulent mixing and efficient heat transfer. Twin Gilson 305 dual piston pumps, (flow rates between 0.5 mL/min and 50 mL/min) provide the solvent and reagent feeds for the reactor system. Figure S2 shows a representative flow reactor configuration for MOF processing. In a typical scale-up reaction, two separate solutions of the precursors are pumped through a T-type static mixer to promote complete mixing of the reagent input streams. The combined reagent streams are then directed into the heated reactor zone of the Salamander Flow Reactor for a predetermined residence time. On exiting the reactor zone, the stream is passed through a back-pressure regulator (up to 20 bar) and then collected into an ice-cooled collection flask (Schott Bottle, 5 L). Figure S2. Photograph of the Salamander Flow Reactor (Cambridge Reactor Design) used for the large-scale (macroscale) production of HKUST-1. The system comprises two HPLC pumps (Gilson) and a 108 mL stainless steel tubing reactor (0.6 mm i.d.) and custom static mixers throughout length of tubing. Figure S3. Representations of BET surface area, SABET respect the different concentrations of copper used for the synthesis of HKUST-1 at 80 ºC (a) and at 140 ºC (b), using different flow rates. Figure S4. SEM image and XRPD patterns of the HKUST-1 crystals synthesized by flow chemistry at 140 ºC and using a flow rate of 20 mL·min-1(green), compared with the simulated XRPD pattern of HKUST-1 (black). This XRPD pattern was collected using Cu Kα radiation. Figure S5. Image showing the amount of HKUST-1 produced during 50 minutes of continuous operation of the larger flow reactor (Salamander). STY of the clean product: 4553 Kg m-3 day-1. The Space Time Yield (STY) was calculated according to the definition: mass (m) of product per volume (V) of reactant mixture and reaction time (t) [day]. A working day was assumed to be 8 hours (480 min). 𝑆𝑇𝑌 = 𝑚[𝑘𝑔] 𝑉[𝑚3 ] 𝑡[𝑑𝑎𝑦] For example for the HKUST-1 MOF we obtain 1.224 g of product using a total 108 ml of reactants in 1.2 min (which is the time it takes to pass for the reactants to flow through the reactor when we use a flow of 90 ml·min-1). We can thus calculate: 1.224 𝑔 106 𝑚𝑙 1 𝑘𝑔 480 𝑚𝑖𝑛 𝑘𝑔 = 4533.3 3 3 108 𝑚𝑙 1.2 𝑚𝑖𝑛 1 𝑚 1000 𝑔 1 𝑑𝑎𝑦 𝑚 𝑑𝑎𝑦 Salamander Figure S6. Experimental N2 isotherms of HKUST-1 at 80 ºC using a flow rate of 2 mL·min-1 (A), 10 mL·min-1 (B), 20 mL·min-1 (C) and 20 mL·min-1 at 140 ºC (D). Experimental N2 isotherms of UiO-66 (E) and NOTT-400 (F). HKUST-1 experimental N2 isotherm at 140 ºC using the Salamander Plug Flow reactor (G). Figure S7. Thermogravimetric analysis of HKUST-1 (A), UiO-66 (B) and NOTT-400 (C) (heating rate: 5 ºC/min). Figure S8. Experimental H2 isotherms of HKUST-1 (A), UiO-66 (B) and NOTT-400 (C) acquired at 77K. Figure S9. Experimental pore size distributions calculated from N2 isotherms at 77 K of HKUST-1. Calculated using the DFT method with slit pore model. Figure S10. Experimental pore size distributions calculated from N2 isotherms at 77 K of NOTT-400. Calculated using the DFT method with slit pore model. Figure S11. Experimental pore size distributions calculated from N2 isotherms at 77 K of UiO-66. Calculated using the DFT method with slit pore model. References 1: Micic, N.; Young, A.; Rosselgong, J.; Hornung, C.H. Scale-up of the Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerization Using Continuous Flow Processing. Processes 2014, 2, 58-70.