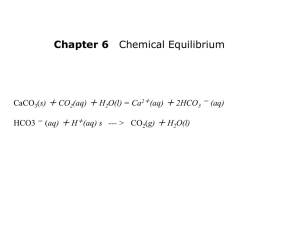
workbook unit 4 equilibrium acids and bases
advertisement

Name:________________________________ _____ Chemistry 30 Equilibrium, Acids and Bases Workbook Chemical Equilibrium 1. For each of the following, write the chemical reaction equation with the appropriate equilibrium arrows. a) pH measurements indicate that acetic acid in vinegar is approximately 1% ionized into hydrogen ions and acetate ions. b) Quantitative analysis of the reaction of sodium sulphate and calcium chloride solutions shows that the products are favoured. c) Aluminum sulphate solution reacts quantitatively with a sodium hydroxide solution. d) An analysis of the reaction between sodium hypochlorite and ammonium nitrate shows that the reactants are favoured. 2. Write the equilibrium law for each of the following chemical reaction equations. a) 2 SO2(g) + O2(g) ⇌ 2 SO3(g) b) 2 NO2(g) ⇌ 2 NO(g) + O2(g) c) N2(g) + 3 H2(g) ⇌ 2 NH3(g) d) Na2SO4(aq) + CaCl2(aq) ⇌ 2 NaCl(aq) + CaSO4(s) 3. Chlorine and carbon monoxide gases are mixed in a 1.00 L container producing COCl2(g). Initially, 1.5 mol of chlorine was present with excess carbon monoxide. The percent reaction is 53%. a) Write the chemical reaction equation including the appropriate equilibrium arrows and the percent reaction. b) Write the equilibrium law for this reaction. c) At equilibrium, 0.80 mol of COCl2(g), 1.75 mol of carbon monoxide and 0.70 mol of chlorine were present. Calculate the equilibrium constant. 4. In an experiment at 200C, 0.500 mol/L of hydrogen bromide gas is placed in a sealed container and it decomposes into hydrogen gas and bromine gas. a) Write the equilibrium equation and law for this reaction. b) The equilibrium concentrations in this system are: [HBr(g)] = 0.240 mol/L, [H2(g)] = [Br2(g)] = 0.130 mol/L. Calculate the equilibrium constant. Chem 30 Equilibrium, Acids & Bases Workbook 1 Le Châtelier’s Principle 1. Nitrogen monoxide, a major air pollutant, is formed in automobile engines from the endothermic reaction of nitrogen gas and oxygen gas. a) Write the equilibrium reaction equation including the term “energy” in the equation. b) What is the direction of the equilibrium shift if the concentration of oxygen is increased? c) What is the direction of the equilibrium shift if the pressure is increased? d) What is the direction of the equilibrium shift if the temperature is decreased? e) Gasoline burns better at higher temperatures. What is one disadvantage of operating automobile engines at higher temperatures? 2. In a sealed container, nitrogen monoxide gas and oxygen gas react to form nitrogen dioxide gas and are allowed to come to equilibrium. The reaction of nitrogen monoxide and oxygen is exothermic. a) Write the equilibrium reaction equation including the term “energy” in the equation. b) What is the direction of the equilibrium shift if the temperature is decreased. c) What is the direction of the equilibrium shift if the [NO(g)] is decreased. d) What is the direction of the equilibrium shift if the [NO2(g)] is increased. e) What is the direction of the equilibrium shift if the volume of the system is decreased. 3. The following is an equilibrium mixture: Fe3+(aq) + SCN(aq) ⇌ FeSCN2+(aq) faint yellow colourless red Predict the colour change in the mixture when each of the following changes is made: a) a crystal of KSCN(s) is added to the system b) a crystal of FeCl3(s) is added to the system c) a crystal of NaOH(s) is added to the system 4. Use Le Châtelier’s Principle to describe the effect of the following changes on the position of the equilibrium: A. The equilibrium is: N2O3(g) ⇌ NO(g) + NO2(g) a) increase the [NO] b) increase the [N2O3] c) add a catalyst d) increase the pressure by decreasing the volume B. The equilibrium is: 2 H2(g) + 2 NO(g) ⇌ N2(g) + 2 H2O(g) a) decrease the [N2] b) decrease the pressure by increasing the volume c) decrease the [NO] C. The equilibrium is: 2 CO(g) + O2(g) ⇌ 2 CO2(g) + 566 kJ a) increase the temperature b) add a catalyst c) increase the [O2] Chem 30 Equilibrium, Acids & Bases Workbook 2 D. The equilibrium is: I2(g) + Cl2(g) ⇌ 2 ICl(g) H = +35.0 kJ a) decrease the temperature b) decrease the [Cl2] c) increase the pressure by decreasing the volume 5. Describe the effect (increase, decrease or no change) on the concentration of the boldfaced substance by each of the given changes. A. The equilibrium is: N2(g) + 3 H2(g) ⇌ 2 NH3(g) a) increase the [N2] b) increase the volume c) increase the temperature d) add a catalyst H = 92.0 kJ B. The equilibrium is: 2 HF(g) ⇌ F2(g) + H2(g) a) decrease the [H2] b) decrease the volume c) decrease the temperature H = +536.0 kJ C. The equilibrium is: SnO2(s) + 2 CO(g) ⇌ Sn(s) + 2 CO2(g) H = +13.0 kJ a) increase the [CO] b) add a catalyst c) increase the temperature Chem 30 Equilibrium, Acids & Bases Workbook 3 Graphical Analysis The Haber-Bosch process of the industrial production of ammonia involves the equilibrium N2(g) + 3 H2(g) 2 NH3(g) + 92.2 kJ. In a laboratory experiment designed to study this equilibrium, a chemical engineer injects 0.20 mol of N2(g) and 0.60 mol of H2(g) into a 1.0 L flask at 500C. She records her analysis of the flask at 5 s intervals in the table shown. Time (s) 0 5 10 15 20 25 N2(g) 0.20 0.14 0.11 0.10 0.10 0.10 Concentration (mol/L) H2(g) 0.60 0.42 0.33 0.30 0.30 0.30 NH3(g) 0.00 0.12 0.18 0.20 0.20 0.20 1. Analyze the data by: a. Draw a graph of the concentrations of N2(g), H2(g) and NH3(g) versus time on the graph paper below. Include a legend with your graph. b. State the time required for equilibrium to be established c. Calculate the equilibrium constant for this reaction…showing all work. Chem 30 Equilibrium, Acids & Bases Workbook 4 2. Show the equilibrium adjustment in each of the following situations graphically: ***Note that the relative positioning of the molecules is not relevant A. The equilibrium is: H2(g) + I2(g) ⇌ 2 HI(g) + 52 kJ a) increase the temperature b) decrease the volume c) inject some H2(g) d) add a catalyst B. The equilibrium is: 2 SO2(g) + O2(g) ⇌ 2 SO3(g) a) inject some SO2(g) b) decrease the temperature c) increase the volume d) increase the [SO3(g)] H = 197.0 kJ C. The equilibrium is: CO(g) + H2O(g) ⇌ CO2(g) + H2(g) a) inject some CO2(g) b) remove some CO2(g) c) increase the temperature d) decrease the pressure by increasing the volume H = 41.0 kJ 3. Interpret the following graphs in terms of the changes which must have been imposed on the equilibrium: A. The equilibrium is: PCl5(g) + 92.5 kJ ⇌ PCl3(g) + Cl2(g) a) b) B. The equilibrium is: H2O(g) + Cl2O(g) ⇌ 2 HOCl(g) + 70 kJ a) Chem 30 Equilibrium, Acids & Bases Workbook b) 5 ICE Tables 1. 1.00 mol of hydrogen gas and 1.00 mol of iodine gas are sealed in a 1.00 L reaction vessel and allowed to react at 450C. At equilibrium, 1.56 mol of hydrogen iodide gas is present. Calculate Kc for the reaction. 2. In an experiment, 2.00 mol of H2(g) and 2.00 mol of F2(g) are introduced into a 1.00 L flask at 500C. After equilibrium was reached, the concentration of HF(g) was 0.500 mol/L. Calculate the Kc for this reaction at 500C. 3. Phosphorus pentachloride gas decomposes into phosphorus trichloride gas and chlorine gas. If the [PCl5(g)]i = 8.1 x 10-3 mol/L and the [PCl3(g)]i = 0.298 mol/L, calculate the Kc. The [Cl2(g)]eq = 2.00 x 103 mol/L. 4. A 1.0 L flask was filled with 2.0 mol of SO2(g) and 2.0 mol of NO2(g) and heated to 250C. If the Kc is 2.75, calculate the equilibrium concentrations of all species at 250C. SO2(g) + ⇌ NO2(g) SO3(g) + NO(g) 5. At a certain temperature, Kc = 2.6 104 for the reaction of H2O with Cl2O. Calculate the equilibrium concentrations of each species if 10.0 g of H2O and 20.0 g of Cl2O are mixed in a sealed 2.00 L flask. H2O(g) + Cl2O(g) ⇌ 2 HOCl(g) pH and pOH 1. Use KW to calculate the [H3O+(aq)] in a solution which has a [OH(aq)] of 1.0 1011 mol/L. 2. Use KW to calculate the [OH(aq)] in a solution which has a [H+(aq)] of 1.0 104 mol/L. 3. Determine the pH from the following [H3O+(aq)] or [OH(aq)]. d) [H3O+(aq)] = 1.0 1013 mol/L pH = __________ acidic or basic ____________ e) [H3O+(aq)] = 1 102 mol/L pH = ___________ acidic or basic ____________ f) [H3O+(aq)] = 106 mol/L pH = ___________ acidic or basic ____________ g) [OH(aq)] = 0.0010 mol/L pH = ___________ acidic or basic ____________ h) [OH(aq)] = 1.0 106 mol/L pH = ___________ acidic or basic ____________ 4. Find the pH of a lime that has [H3O+(aq)] = 0.0120 mol/L. 5. Determine the pH of a blood sample with a [OH(aq)] = 2.6 107 mol/L. 6. An ammonia solution has a pOH of 2.92. What is the concentration of hydroxide ions in solution? 7. Find the [H3O+(aq)] and pH of a solution made by dissolving 10.0 g of KOH in water to make 4.00 L of solution. Chem 30 Equilibrium, Acids & Bases Workbook 6 8. A sodium hydroxide solution is prepared by dissolving 2.50 g to make 2.00 L of solution. Calculate both the hydroxide ion and hydrogen ion concentrations. 9. A 0.728 g sample of hydrogen chloride gas is dissolved in 200 mL of water. Calculate both the hydroxide ion and hydrogen ion concentrations. 10. Calculate the pH of a solution made by dissolving 7.50 g of strontium hydroxide to make 500 mL of solution. 11. Complete the following table: [OH(aq)] [H3O+(aq)] pH pOH Acid/Base/Neutral 4.0 x 106 mol/L 9.50 2.0 1011 mol/L 10 mol/L 5.563 1.36 Brønsted-Lowry Acids and Bases 1. Label each reactant in the following equations as Brønsted-Lowry acid or base: a) HSO3(aq) b) NH3(aq) c) HF (aq) d) H2SO3(aq) + + ⇌ H2O(l) H2O(l) ⇌ ⇌ HSO3(aq) + + HS (l) Chem 30 Equilibrium, Acids & Bases Workbook ⇌ 7 H3O+(aq) + SO32(aq) NH4+(aq) + OH(aq) F(aq) + H2SO3 (aq) HSO3(aq) + H2S (aq) 2. For each of the following reactions, label each reactant as Brønsted-Lowry acid or base and complete the reaction. Cl(l) a) HNO2 (aq) + b) CH3COOH (aq) + c) H2S (aq) NO2(aq) d) HCO3(aq) e) CO32(aq) + SO42(aq) + ⇌ ⇌ + + S2 (l) ⇌ + H2O(l) ⇌ + + + ⇌ 3. For questions 1 and 2, label each product as conjugate acid or conjugate base. Strengths of Acids 1. Calculate the pH of 500 mL of each of the following acids: a) 1.0 102 mol/L HCl(aq) b) 6.00 mol/L HNO2(aq) c) 1.50 mol/L H2SO3(aq) d) 6.8 102 mol/L HNO3(aq) e) 6.3 101 mol/L HF(aq) 2. A 0.80 mol/L solution of an unknown acid, HX(aq), has a pH of 3.75. a) Calculate the % reaction. b) Calculate the Ka, the acid ionization constant. 3. Calculate the pH of a solution containing 0.25 mol/L of an acid with an acid ionization constant (Ka) of 3.2 106 mol/L. Chem 30 Equilibrium, Acids & Bases Workbook 8 Strengths of Bases 1. Calculate the pH and pOH for each of the following: a) 1.00 mol/L NaOH(aq) b) 1.00 mol/L Ca(OH)2(aq) c) 0.650 mol/L Al(OH)3(aq) d) A solution made by dissolving 5.82 g of barium hydroxide in 2.00 L of water. 2. Calculate the Kb for each of the following bases at 25C: a) NO2(aq) b) F(aq) c) HSO3(aq) d) HCO3(aq) e) OOCCOO2(aq) 3. Calculate the pH of a 13.5 mol/L solution of H2PO4(aq) using the following reaction: H2PO4(aq) + H2O(l) ⇌ H3PO4(aq) + OH(aq) Strengths of Acids and Bases – pH Calculations Calculate the pH for each of the following solutions. Show all work. 1. 0.32 mol/L Mg(OH)2(aq) 2. 6.00 mol/L NH3(aq) 3. 2.0 104 mol/L KHSO4(aq) **red with both litmus 4. 3.0 mol/L H2S(aq) 5. 0.750 mol/L KF(aq) 6. 0.0505 mol/L HI(aq) 7. 16 mol/L CH3COOH(aq) 8. 2.00 mol/L NaCN(aq) 9. 5.7 mol/L Na2HPO4(aq) **blue with both litmus 10. 0.750 mol/L Al(OH)3(aq) Chem 30 Equilibrium, Acids & Bases Workbook 9 Predicting Acid-Base Reactions For each of the following problems: 1. Write a reaction equation. 2. Label reactants as acid or base and products as conjugate acid or conjugate base. 3. Use appropriate arrow notation to indicate reaction predomination (forward or reverse). 1. Solutions of Na2SO3(aq) and HF(aq) are mixed in a beaker. 2. A solution of NH4NO3(aq) and a solution of NaCH3COO(aq) are mixed. 3. Sodium benzoate is often used as a preservative. Write the equation for NaC6H5COO(s) dissolving in a solution of NaHSO4(aq). 4. A household ammonia solution is mixed with a solution of nitrous acid. 5. Nitric acid and potassium hydroxide solutions are mixed. 6. Sodium sulphate is dissolved into a solution of sulphurous acid. 7. Ammonium fluoride is dissolved in water. 8. Benzoic acid can be used as a starting material for the synthesis of aspirin. Write an equation for the reaction of benzoic acid with water. 9. In solution, the poisonous gas H2S(g), which is sometimes found in natural gas, reacts with carbonate ions. 10. Rain water in an area near a tar sands plant could dissolve SO2(g). Write the equation that represents the reaction of this acid solution with sodium sulphate. 11. Vinegar, a dilute ethanoic acid solution, is used to neutralized some spilled lye (sodium hydroxide) 12. Sodium hydrogen carbonate may be used directly or in gripe water to neutralize excess stomach acid, HCl(aq). 13. Sodium hydrogen carbonate is mixed with a solution of potassium dihydrogen phosphate. Chem 30 Equilibrium, Acids & Bases Workbook 10 Polyprotic Acids and Bases For each of the following problems, write all reaction steps and the net reaction. Assume all reactions are quantitative. 1. Potassium hydroxide is continuously added to a carbonic acid solution. 2. Lithium hydroxide is continuously added to a citric acid solution, C3H4OH(COOH)3(aq). Citric acid can be abbreviated H3Ct(aq). 3. Potassium hydroxide is continuously added to sulphuric acid until no further reaction occurs. 4. A sodium carbonate solution is titrated with hydrobromic acid. 5. A solution of sodium hydrogen phosphate is titrated with hydroiodic acid. pH Curves For each of the following curves state: 1. the titrant 2. what type of sample is being used eg) monoprotic acid, polybasic substance etc. 3. the endpoint pH value(s) 4. potential indicator for each endpoint 5. the equivalence point(s) Titration Curve 1. Titration Curve 12 10 pH 8 6 4 2 2. - 12 10 pH 50 100 150 50 volume of titrant added (mL) Chem 30 Equilibrium, Acids & Bases Workbook 8 6 4 2 100 150 volume of titrant added (mL) 11 Titration Curve 3. Titration Curve 12 - pH 10 8 6 4 2 4. - pH 50 100 150 10 8 6 4 2 50 100 150 volume of titrant added (mL) volume of titrant added (mL) 5. 12 - Titration Curve pH 12 10 8 6 4 - 2 50 100 150 volume of titrant added (mL) Buffers 1. One of the most important buffers in our bodies is the carbonic acid – hydrogen carbonate ion system. a) Write the equation for the reaction that occurs when a strong acid is added to this buffer. b) Write the equation for the reaction that occurs when a strong base is added to this buffer. 2. Another important buffer in our bodies is the dihydrogen phosphate – hydrogen phosphate ion system. a) Write the equation for the reaction of this buffer with ammonia. b) Write the equation for the reaction of this buffer with carbonic acid. Chem 30 Equilibrium, Acids & Bases Workbook 12 Properties of Strong and Weak Acids and Bases 1. Fill in the chart of expected results for acids that all have a concentration of 0.1 mol/L. Acid Chemical pH Conductivity Reactivity with formula (slightly less (high or low) magnesium metal than 7 or much (high or low) less than 7) hydrochloric acid ethanoic acid boric acid hydrofluoric acid sulfuric acid HClO4(aq) H3PO4(aq) HBr(aq) H2SO3(aq) 2. Two different acidic solutions have a concentration of 0.1 mol/L. Solution A conducts electricity extremely well, while solution B conducts very poorly. Which of the solutions will have a lower pH? Explain using a description of what is happening on a molecular level. 3. You have two basic solutions. One has a concentration of 1.0 mol/L and one has a concentration of 0.1 mol/L. You know one is a strong base and one is a weak base. Can you determine which solution is which based on pH? If so, explain how. If not, explain why not. Chem 30 Equilibrium, Acids & Bases Workbook 13 Acid-Base Indicators Use your table of indicators, located on p. 230 of the text (and in your notes), to answer the following questions: 1. Fill in the spaces in the following table: pH colour of colour of orange IV bromocresol green 1.0 colour of bromothymol blue colour of phenol red 4.2 5.8 9.0 2. Use the results displayed below to determine the pH ranges of the solutions. Solution colour of colour of colour of bromocresol bromothymol blue phenolphthalein green A yellow yellow colourless B blue blue pink C blue blue colourless D green yellow colourless pH 3. What indicator could you use to distinguish between two solutions, one that has a pH of 8 and one that has a pH of 11? 4. Using three indicators, design a procedure that would be able to identify four solutions that have pHs of 3, 6, 8, and 11, respectively. 5. What colour would bromocresol green be in an acid solution made by dissolving 4.3 g of pure hydrochloric acid in 20 L of water? Chem 30 Equilibrium, Acids & Bases Workbook 14 Calculating pH after Dilution 1. A 35.0 mL volume of a 0.489 mol/L solution of hydrochloric acid is diluted to a volume of 300 mL. (a) What is the pH of the concentrated solution? (b) What is the concentration of the diluted solution? (c) What is the pH of the diluted solution? (d) Compare your answers to (a) and (c). Considering the acid solution has been diluted, do your answers make sense? 2. A 50 mL volume of a 0.7983 mol/L solution of sodium hydroxide, NaOH(aq), is diluted to a volume of 1.50L. (a) What is the pOH of the concentrated solution? (b) What is the pH of the concentrated solution? (c) What is the concentration of the dilute solution? (d) What is the pOH of the dilute solution? (e) What is the pH of the dilute solution? (f) Compare your answers for (b) and (e)? Do they make sense considering the basic solution was diluted? Chem 30 Equilibrium, Acids & Bases Workbook 15 3. A 20 mL volume of a 3.52 10–3 mol/L solution of nitric acid is diluted to a volume of 25 L. What is the pH of the diluted solution? 4. A concentrated solution is made by dissolving 3.5 g of hydrobromic acid in 20.0L of water. A 50.0 mL volume of the concentrated solution is then used to make 100 L of a new solution. What is the pH of the new solution? Chem 30 Equilibrium, Acids & Bases Workbook 16