a cause of post-operative non-cardiogenic pulmonary oedema
advertisement

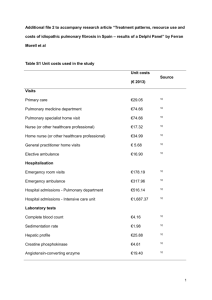
CASE REPORT SUBSTANDARD NEOSTIGMINE: A CAUSE OF POST-OPERATIVE NON-CARDIOGENIC PULMONARY OEDEMA Ullhas S. Misal1, Sudhir G. Chavan2, Vikas A. Kurhekar3 HOW TO CITE THIS ARTICLE: Ullhas S. Misal, Sudhir G. Chavan, Vikas A. Kurhekar. “Substandard Neostigmine: A Cause of Post-Operative Non-cardiogenic Pulmonary Oedema”. Journal of Evidence Based Medicine and Healthcare; Volume 1, Issue 9, October 31, 2014; Page: 1206-1211. ABSTRACT: Cases of Non-cardiogenic pulmonary edema occurred at Government Medical College, Miraj and PVP General Hospital, Sangli during the month of December 2007 after reversal of neuromuscular block with Neostigmine resulting in morbidity. The drug was newly supplied on a rate contract basis to Government hospitals. The incidence was reported to the authorities. Further use of the drug stopped and the sample sent to Food and Drugs Administration department (FDA) for analysis. FDA analyzed the drug and reported that it was of a “Substandard Quality”. We are presenting 2 cases of Non-cardiogenic pulmonary edema occurred after the use of this ‘Substandard Neostigmine’. KEYWORDS: Neostigmine, Drug related pulmonary edema, reversal of neuromussular block. INTRODUCTION: Neostigmine is the most commonly used anticholinesterase agent to reverse residual neuromuscular block at the end of surgery. It inhibits the normal hydrolysis of acetylcholine and thus raises its concentration at cholinergic nerve endings. The drug increases both muscarinic and nicotinic effects of acetylcholine. The muscarinic effects include bradycardia, hypotension, profuse salivary and bronchial secretions, bronchospasm, and stimulation of smooth muscles particularly of hollow viscera. In presence of respiratory acidosis the drug may cause severe dysrhythmias. It should be used with caution in patients with asthma and heart disease. Neostigmine overdose may cause restlessness, weakness, muscular twitching, sweating, salivation, nausea, vomiting, pin point pupil, colic, bronchospasm, and hypotension etc. Aspiration of stomach contents, trauma, septicaemia, drug allergies are well recognized causes of Non-cardiogenic pulmonary edema after general anaesthesia. Non-cardiogenic pulmonary edema occurred after reversal of neuromuscular block with neostigmine at “Government Medical College and PVP General Hospital, Miraj-Sangli” during December 2007. In this case report, we are presenting two cases of Non-cardiogenic pulmonary edema occurred after reversal of neuromuscular block with neostigmine. CASE 1: A middle aged male with h/o duodenal ulcer was posted for gastrojejunostomy. Preanesthesia findings revealed h/o vomiting on and off, pain in abdomen, repeated use of NSAIDs and chronic alcoholism. His biochemical profile, ECG and CXR show no abnormality. His PR was 88 beats/min, and BP was 98/68 mmHg, and chest auscultation was normal. Patient’s vital parameters were stable throughout intraoperative period and his urine output was 150 ml. At the end of the surgery, his neuromuscular block was reversed with Inj Neostigmine 2.5 mg and Inj Glycopyrrolate 0.4 mg IV. His trachea was extubated after complete recovery and he J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/ Issue 9 / Oct. 31, 2014. Page 1206 CASE REPORT was shifted to recovery room for observation. In recovery room, we noticed tachycardia (PR136/min), tachypnoea (RR-32/min), mild to moderate cyanosis and air hunger in the patient. His BP was 66/40 mmHg and SpO2 84 %. Chest auscultation showed bilateral crepitation. Immediate re intubation and ventilation with 100 % oxygen supplementation was carried out. Inj mephentermine 15 mg IV given along with Inj Dexamethasone (8mg) and Hydrocortisone (100mg), and when BP became normal (106/60 mmHg), Inj lasix 20+20 mg given IV. Inj deriphylline 2 ml IV given. He was re paralyzed with Inj Vecuronium 4 mg and was shifted to ICU where he was put on ventilator for next 12 hrs. In due course of time, he improved symptomatically and clinically. Gradual weaning from ventilator done and he was extubated next day without any sequel. CASE 2: A middle aged female weighing 50 kg and ASA status I with fracture left radius-ulna was posted for plating. I/O: PR 80-104/min, BP 110-134 mmHg, RR 14-16/min controlled, SpO2 98-99 %. P/O: Reversal with Inj Neostigmine 2.5 mg, Glycopyrrolate 0.4 mg, extubation after adequate recovery. IN RECOVERY ROOM: Patient c/o dyspnoea, restlessness, nausea. There was mild cyanosis, PR 126/min, BP 80/60 mmHg, RR-32/min and SpO2 86 %. On auscultation there were bilateral basal crepts. Patient re intubated and re-paralyzed by giving Inj Vecuronium 4 mg. Ventilated with 100 % oxygen. Inj Dexamethasone and Hydrocortisone were given along with Aminophylline 250 mg bolus. IV Hydroxyethyl starch pushed fast (300 ml), Inj Lasix 40+20+20=total 80 mg, Dopamine drip started to maintain BP above 90 mmHg. Patient subsequently improved symptomatically and clinically in next 3 hrs and shifted to ICU where he was put on mechanical ventilator. He was weaned gradually from ventilator and extubated next day and shifted to the ward without any respiratory problem. Drug Dose Glycopyrrolate 0.2 mg Midazolam 0.03 mg/kg Pentazocine 0.3 mg /kg Pentothal Sodium 5 mg/kg Suxamethonium 2 mg/kg Vecuronium Bromide 0.08 – 0.1 mg/kg Halothane 0.4-0.6 % Neostigmine 0.05 mg/kg Glycopyrrolate 0.004 mg/kg Table No. 1: Showing the drugs used for Induction, Maintenance and Reversal of G.A. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/ Issue 9 / Oct. 31, 2014. Page 1207 CASE REPORT Fig. 1: CXR P-A view showing pulmonery oedema These events compelled us to find out the cause of this morbidity. We have had a long discussion amongst the departmental colleagues and surgeons and physician. Consensus was reached that the inj Neostigmine should be the most probable drug responsible for this. We stopped the use of that particular batch of Neostigmine (Batch No. 02288) and the incidence was reported to the authorities. Samples were preserved and sent to the FDA department for analysis. Fig. 2: Reporting of the complication to the authorities J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/ Issue 9 / Oct. 31, 2014. Page 1208 CASE REPORT Similar cases were also reported from Sir JJ group of Hospitals, Mumbai from use of the same batch. FDA analyzed the drug and reported that the drug supplied was of a substandard quality. Fig. 3: Analysis report received from FDA DISCUSSION: According to literature, neostigmine overdose may cause acute cholinergic crisis characterized by increased salivation, bronchial secretions, bronchospasm, tachy or bradycardia and hypotension. Sometimes agitation, fear or restlessness may occur and death may result from cardiac arrest or respiratory insufficiency and pulmonary edema. In our patients the dose of neostigmine used was 0.05mg/kg body weight which rules out dose related complications. Neostigmine is known to cause various arrhythmias in patients with acidosis or in patients with asthma or heart disease. But none of our patients were having asthma and symptomatic cardiac disease. Negative pressure pulmonary edema,(2,3,4) an uncommon problem of persistent inspiratory efforts against an obstructed airway was ruled out in our patients as all of them were extubated after adequate recovery from their residual neuromuscular block and there was a good air blast seen before we removed the tube. Raiger et al(1) reported two cases of non-cardiogenic pulmonary edema occurred after reversal of neuromuscular block with neostigmine. This was questioned by Mukul Kapoor.(5) In our case, it was the substandard quality of drug which caused post reversal non-cardiogenic pulmonary edema. Drug induced pulmonary edema (6,9,10,11,12) has also been documented with several agents that have different pharmacological effects. To mention few are Salicylates, Naloxone, Tocolytics, Protamine, Amiodarone, Insulins, Streptokinase, Hydrochlorothiazide etc. Non-cardiogenic pulmonary edema has been reported after administration of Propofol and Ondansetron. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/ Issue 9 / Oct. 31, 2014. Page 1209 CASE REPORT The diagnosis of non-cardiogenic pulmonary edema is done by exclusion, as no specific test is available. In our patients the incidence occurred just after reversal of residual neuromuscular block with neostigmine and glycopyrrolate. Of these, glycopyrrolate was used at induction hence the most probable agent should be neostigmine. Another question was why only these patients suffered when the GA was given to many. After detailed enquiries amongst staff and residents, we came to know that these were the only patients in whom all the ampoules used were of the same batch. We were having old stock of neostigmine and mixed batch ampoules were used for reversal in others due to which other patients were not suffered. Non-cardiogenic pulmonary edema can well be managed upon prompt recognition and intense supportive therapy although mortality has been reported. Early ventilator support with or without muscle paralysis reduces the work of breathing and is a key to success. CONCLUSION: Whenever you are using a drug from an unfamiliar company, be alert and watchful than usual. Substandard drugs may cause any complication. If you are suspicious about any drug, stop using it. Inform immediately to the concerned authorities. Preserve samples and send them to FDA department for analysis to avoid complications elsewhere. Monitor the patients in the recovery room at least for 45-60 min after reversal. Do not hesitate to re intubate and paralyze the patient if there is respiratory inadequacy. Elective ventilation will reduce the work of breathing and thereby further complications. Whenever there is a problem, call for help. Discuss the problem amongst fellow colleagues; some of them may give helpful advice. REFERENCES: 1. Raiger LK, Naithani U, Vijay BS, Gupta P, Bhargava V. Non-cardiogenic pulmonary oedema after neostigmine given for reversal: A report of two cases; Indian J Anaesth 2010; 54: 33841. 2. Goli AK, Goli SA, Byrd Jr, Roy TM. Spontaneous negative pressure changes: An unusual cause of non-cardiogenic pulmonary edema. J Ky Med Assoc. 2003; 101: 317-20. 3. Sharma S, Jain A. Negative pressure pulmonary edema after septoplasty. Internet J Anaesthesiol 2010; ISSN: 1092-460X. 4. Stuth EA, Stucke AG, Berens RJ. Negative pressure pulmonary edema in a child with hiccups during induction. Anaesthesiology 2000; 282-4. 5. Mukul Kapoor. Could pulmonary oedema be attributed to the use of neostigmine? Indian J Anaesth. 2010; 54 (6): 582. 6. Reed CR, Glauser FL. Drug induced noncardiogenic pulmonary edema. Chest. 1991; 1120-4. 7. Kallet RH, Daniel BM, Gropper M, Mathay MA. Acute pulmonary edema following upper airway obstruction: Case reports & brief review. Respir Case. 1998; 43: 476-80. 8. Ohmi S, Takei T, Habuka K, Watanabe Y. Acute pulmonary capillary leak syndrome during elective surgery under general anaesthesia. J Anesth 2008; 22: 77-80. 9. Inal MT, Memis D, Vaton I, CCakir U, Yildiz B. Late onset pulmonary edema due to propofol. Acta Anesthesiologica Scandinavica 2008; 52: 1015-7. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/ Issue 9 / Oct. 31, 2014. Page 1210 CASE REPORT 10. Gamboa PM, Achotegui V, Irigoyen J, Perez-Asenjo J, Merino J, Sanz ML. Hydrochlorothiazide-induced acute non-cardiogenic pulmonary edema. J Investig Allergol Clin Immunol 2005; 15: 299-301. 11. Gupta A, Verma K. Ondansetron: An acute fulminant pulmonary edema. Ind J Anaesthesiology 2009; 53: 240-1. 12. Briasoulis E, Pavlidis N. Non-cardiogenic pulmonary edema: An unusual and serious complication of anti-cancer therapy. Oncologist 2001; 6: 153-61. AUTHORS: 1. Ullhas S. Misal 2. Sudhir G. Chavan 3. Vikas A. Kurhekar PARTICULARS OF CONTRIBUTORS: 1. Professor, Department of Anaesthesia, Government Medical College and Hospital, CVTS Department, Aurangabad, Maharastra. 2. Professor, Department of Anaesthesia, Government Medical College and Hospital, CVTS Department, Aurangabad, Maharastra. 3. Associate Professor, Department of Anaesthesia, Government Medical College and Hospital, CVTS Department, Aurangabad, Maharastra. NAME ADDRESS EMAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ullas S. Misal, Flat No. S-1, Venkatesh Residency, C. S. No. 246 A/B, Behind Kelavkar Hospital, Nagala Park, ‘E’-ward, Kolhapur-416003. E-mail: ullhasmisal@gmail.com Date Date Date Date of of of of Submission: 08/09/2014. Peer Review: 09/09/2014. Acceptance: 06/10/2014. Publishing: 23/10/2014. J of Evidence Based Med & Hlthcare, pISSN- 2349-2562, eISSN- 2349-2570/ Vol. 1/ Issue 9 / Oct. 31, 2014. Page 1211