Chapter 10.1b Guided Notes – Radioactive Decay Calculations
advertisement

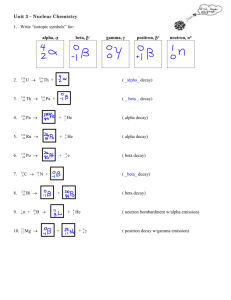
Chapter 10.1b Guided Notes – Radioactive Decay Calculations 1. What happens during alpha decay? 2. How do you symbolize this? 3. What is the starting (reactant) isotope known as? 4. What is the ending (product) isotope known as? 5. Write the nuclear equation for alpha decay of uranium-238: 6. Mass number on left is equal to? 7. Atomic number on left is equal to? 8. How do we find the “daughter” if we are given the “mother”? 9. What happens during beta decay? 10. How do you symbolize this? 11. How does the mother change to daughter in beta decay? 12. Write the nuclear equation for beta decay of thorium-234 13. What happens during gamma decay? 14. How do you symbolize this? 15. How does gamma decay affect the atomic mass and atomic number of the isotopes? 16. When does gamma decay often occur? 17. Write the nuclear equation for thorium-234 as it emits both beta particles and gamma rays: 18. Balancing equations - Alpha Decay of Po-210: a. Atomic Mass: b. Atomic Number: c. New Element: 19. Write a balanced nuclear equation for the alpha decay of thorium-232. 20. Write a balanced nuclear equation for the beta decay of carbon-14. 21. Determine the “daughter” (product isotope) of americium-241 alpha decay. 22. Determine the “daughter” (product isotope) of strontium-90 beta decay.