Name: Date: Reaction Rates 2 Catalyst: Answer the catalyst
advertisement

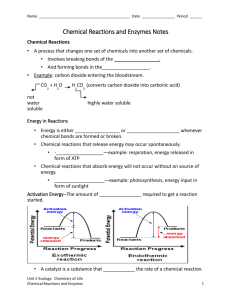
Reaction Rates 2 Name: __________________________ Date: __________________________ Catalyst: Answer the catalyst questions here: Video Investigation: Introduction to Enzymes and Catalysts: What is an enzyme? Why do we need them? What are the four ways that enzymes speed up chemical reactions? Notes: Collision Theory of Reactions In order to react molecules and atoms must collide. They must collide with enough _____________ and at the correct angle to make a reaction happen. _____________________ is the minimum amount of energy required to start a chemical reaction. What is a catalyst and how does it affect reaction rate? We know that increasing temperature, reactant concentration, surface area, and pressure all can _______________ the rate of a chemical reaction. A catalyst is a substance that _______________ the reaction rate when it is added to a reaction mixture. Catalysts increase the reaction rate by ________________________________________. A catalyst is ___________________________________ because it is not used up or changed during the reaction. An enzyme is a ______________________ that acts as a catalyst. It is a biological catalyst. Check for Understanding: A scientist performed the following chemical reaction for the decomposition of hydrogen peroxide: 2 H2O2 2 H2O + O2 In the first trial, oxygen was produced at a rate of 0.001 mol/min. In the second trial, oxygen was produced at a rate of 2 mol/min. Question: Which trial used a catalyst? How do you know? 1 Practice: Read the article and answer the questions. Use complete sentences. Name: __________________________ Date: __________________________ News Article: Without enzyme catalyst, slowest known biological reaction takes 1 trillion years. By LESLIE H. LANG University of North Carolina School of Medicine ---May 5, 2003 All biological reactions within human cells depend on enzymes. Their power as catalysts enables biological reactions to occur usually in milliseconds. But how slowly would these reactions proceed spontaneously, in the absence of enzymes – minutes, hours, days? And why even pose the question? One scientist who studies these issues is Dr. Richard Wolfenden, professor of biochemistry and biophysics and chemistry at the University of North Carolina at Chapel Hill. In 1998, he reported a biological transformation deemed "absolutely essential" in creating the building blocks of DNA and RNA would take 78 million years in water without an enzyme. In 2003, Wolfenden found a reaction that is 10,000 times slower than that. "Its half-time – the time it takes for half the substance to be consumed – is 1 trillion years, 100 times longer than the lifetime of the universe. Enzymes can make this reaction happen in 10 milliseconds." Wolfenden’s research report highlights the catalytic power of certain enzymes that increase the reaction rate of the transformation of biochemicals involved in cell signaling pathways. These enzymes help regulate communication between the body’s cells. Without the enzyme, the uncatalyzed reaction takes 1 trillion years. "This number puts us way beyond the known universe in terms of slowness," he said. "(The enzyme reaction) is 21 orders of magnitude faster than the uncatalyzed case.” Why would we want to know the rate of a biological reaction in the absence of an enzyme? That information would allow biologists to appreciate what natural selection has accomplished over the millennia in the evolution of enzymes as prolific catalysts, Wolfenden said. It also would enable scientists to compare enzymes with artificial catalysts produced in the laboratory. "Without catalysts, there would be no life at all, from microbes to humans," he said. "It makes you wonder how natural selection operated in such a way as to produce a protein that got off the ground as a primitive catalyst for such an extraordinarily slow reaction." Experimental methods used to observe very slow reactions can generate important information for drug design. "Enzymes that do a prodigious job of catalysis are, hands-down, the most sensitive targets for drug development," Wolfenden said. "The enzymes we studied in this report are fascinating because they exceed all other known enzymes in their power as catalysts. We’ve only begun to understand how to speed up reactions with chemical catalysts, and no one has even come within shouting distance of producing their catalytic power." Wolfenden’s research has influenced rational drug design; findings from his laboratory helped spur development of ACE inhibitor drugs, now widely used to treat hypertension and stroke. Questions 1. According to the article, what is activation energy? Based on the article, how does activation energy affect the rate of a chemical reaction? 2. According to the article, what do enzymes do? Why are enzymes important to cellular processes and life? 2 Name: __________________________ Date: __________________________ Practice Instructions: Draw a reaction energy diagram for the breakdown of complex carbohydrates, shown in the box below. This chemical reaction happens in your mouth. It is aided by an enzyme catalyst called amylase. In your diagram, be sure to: (1) label all the axes, (2) include a title, (3) label the reactants and products, (4) draw and label the energy of reaction without the enzyme, (5) draw the energy of reaction with the enzyme, and (6) label the activation energy. amylase complex carbohydrates simple sugars Additional Practice: Reaction rate can be measured by I. rate of formation of the products II. rate of change in mass of reactants plus products III. rate of disappearance of the reactants (a) I only (c) I and II (b) I and III (d) I, II, and III Which of the following is a unit of reaction rate? (a) g/mol (c) mol/L (b) cm/s (d) M/s Which statement describes the effect of concentration on reaction rate? (a) Reaction rates increase as products increase. (b) Reaction rates increase as products increase. (c) Reaction rates increase as reactants increase. (d) Reaction rates increase as reactants decrease. How does increasing temperature increase reaction rate? (a) It increases the concentration of the reactants. (b) It increases the collisions between molecules. (c) It increases the pressure on the reaction. (d) It increases the fraction of molecules that have enough energy to react. A chemical reaction produces 100 mg of a substance per second at 25°C. If doubling the temperature increases the rate of production by a factor of 2, how many milligrams would be produced per second at 50°C? (a) 50 mg (c) 200 mg (b) 100 mg (d) 400 mg 3