Protocol Deviation Reporting Form - Research Ethics
advertisement

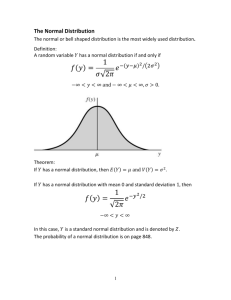
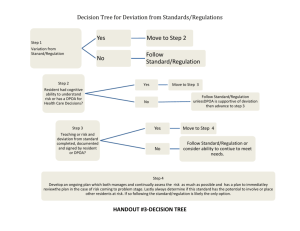
ST. MICHAEL’S RESEARCH ETHICS BOARD (REB) PROTOCOL DEVIATION REPORTING FORM For REB Use Only ST. MICHAEL’S RESEARCH ETHICS BOARD (REB) PROTOCOL DEVIATION REPORTING FORM See ‘Guidelines for Reporting Protocol Deviations to the SMH REB’ and this Reporting Form at: www.stmichaelshospital.com/research/reb.php Complete this form and submit two (2) hard copies to the SMH REB. Submissions that are incomplete will be returned to the submitter. REB #: Study Title: SMH Principal Investigator: Sponsor #: Definition: A Protocol Deviation is an unanticipated or unintentional divergence or departure from the expected conduct of an approved study that is not consistent with the current approved research protocol, consent document or study addenda. Deviations may or may not have a significant effect on the research participant’s rights, safety or welfare, or on the integrity of the data. Deviations are different from amendments in that they generally apply to a single occurrence or participant and are not intended at the time to modify the entire protocol. Note: The term ‘protocol deviation’ and ‘protocol violation’ may be used interchangeably. TYPE of Protocol Deviation:(check all that apply) change in study procedure(s) initiated to eliminate immediate hazards to research participants enrolment of a research participant who did not meet all protocol inclusion/ exclusion criteria, whether agreed to or not by the study Sponsor over-enrolment (exceeding the target number of participants approved by the REB) deviation in the consent process (i.e., failure to obtain informed consent, use of an invalid consent form, missing date of consent, missing signature) performance of a study procedure not approved by the REB failure to perform a required study procedure that, in the opinion of the Principal Investigator, may affect participant safety or data integrity study procedure (i.e., study visit) performed outside of the required timeframe that, in the opinion of the Principal Investigator, may affect participant safety or data integrity study drug/intervention errors (i.e., incorrect study drug/intervention, incorrect dosage of the study drug) breach of confidentiality whereby a research participant’s personal health information (PHI) is revealed to a person without a need to know, or by data exposure (i.e., digital device security breach, documents containing PHI are left unsecured) Protocol Deviation Reporting Form Version Date: 01-Jun-2015 Page 1 of 3 ST. MICHAEL’S RESEARCH ETHICS BOARD (REB) PROTOCOL DEVIATION REPORTING FORM Date of Report (dd/mmm/yyyy) : Name of Protocol Deviation : Type of Report: Initial Follow-Up Final If a follow-up or final report, please indicate the REB submission date(s) of previous report(s) (dd-mmm-yyyy) : Participant Study ID #: Date of Protocol Deviation (dd-mmm-yyyy): Age (years) at time of event: Gender: Date SMH Study Team became aware of Protocol Deviation (dd-mmm-yyyy): Describe the Protocol Deviation and Reason (Please provide a detailed description of the specific protocol deviation, including an explanation for the reason of its occurrence and details of any communications with the sponsor regarding this deviation.) Corrective Actions and Future Actions to Prevent RE-Occurrence (Please provide a detailed description of how the event was handled including any corrective actions and any plans to prevent re-occurrence.) Participant’s outcome of the event (if known). PROTOCOL DEVIATION NOTIFICATION Has the study Sponsor been notified of the event? If no, please explain: YES NO YES NO Was/were the research participant(s) informed of the event? If no, please explain: IMPACT ASSESSMENT Does the Protocol Deviation impact the research participant’s rights, safety, or well-being? Does the Protocol Deviation compromise the scientific integrity of the entire study? Is the Protocol Deviation repetitive in nature and affects the research participant’s rights, safety, or well-being or the scientific integrity of the entire study? Does the Protocol Deviation require change(s) to the study protocol? If yes, submit the changes using the ‘Amendment and Administrative Change Request Form’. Does the Protocol Deviation require change(s) to the consent form(s)? If yes, submit the changes using the ‘Amendment and Administrative Change Request Form’. Did this Protocol Deviation result in a Serious Adverse Event (SAE) / Unanticipated Problem? If yes, submit the SAE/Unanticipated Problem using the ‘Local SAE / Unanticipated Problem Reporting Form’. Principal Investigator Comments: Protocol Deviation Reporting Form Version Date: 01-Jun-2015 Page 2 of 3 ST. MICHAEL’S RESEARCH ETHICS BOARD (REB) PROTOCOL DEVIATION REPORTING FORM DECLARATION BY PRINCIPAL INVESTIGATOR I attest that I as the Principal Investigator (PI) have reviewed the protocol deviation and its safety implications, assessed the relationship of the protocol deviation to the research study and attest to the accuracy of this report. I warrant that this study will continue to be conducted in accordance with the Tri-Council Policy Statement Ethical Conduct for Research Involving Humans (TCPS), the Ontario Personal Health Information Protection Act (PHIPA) 2004, the St. Michael’s Hospital By-laws, the Catholic Association of Canada Health Ethics Guide, and other relevant laws, regulations or guidelines, [e.g., Health Canada Part C, Division 5 of the Food and Drug Regulations, Part 4 of the Natural Health Products Regulations, Medical Devices Regulations, and ICH/GCP Consolidated Guideline E6]. Printed Name of SMH Principal Investigator Signature Date TO BE COMPLETED BY THE RESEARCH ETHICS BOARD I acknowledge that the St. Michael’s Hospital Research Ethics Board has reviewed the documents listed above. Printed Name of REB Member No further action required Protocol Deviation Reporting Form Version Date: 01-Jun-2015 Further action required Signature Date Page 3 of 3