Unit 3.3 Chemical Formulas and Molecular Models
advertisement

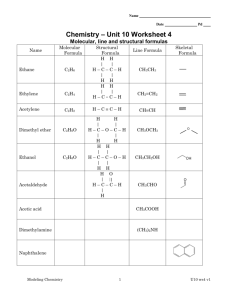
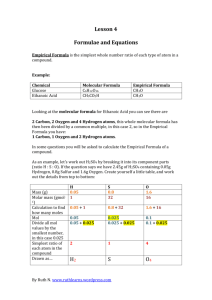
Unit 3.3 Chemical Formulas and Molecular Models 15 minutes – Types of chemical formulas The quickest and easiest way to represent a compound is with a chemical formula. Chemical formulas indicate what elements are present in a compound and how many of each type of element there are. A good example of a chemical formula is the chemical formula for water Chemical symbol for Hydrogen H2O Chemical symbol for Oxygen Subscripts indicate how many of each type of atom are present. In this case the 2 represent two hydrogen atoms. If there is no subscript, then it is understood as one atom. So there is only one oxygen. Chemical formulas usually list the more metallic and or the most positive ions first. In the case of H2O neither element is a metal, but hydrogen forms a (+) cation and oxygen forms a (-) anion, that is why Hydrogen is listed first. In the chemical compound NaCl, sodium is a metal and forms a cation, chlorine is a nonmetal that forms an anion, so sodium is listed first. There are various types of chemical formulas. Empirical formulas provides the relative number of atoms of each element in a compound. Molecular formulas provide the actual number of atoms of each of the elements in a molecule or compound. For example the empirical formula for hydrogen peroxide is HO. This means that for every one Hydrogen there is one oxygen, the molecular formula for hydrogen peroxide is H2O2 and shows the actual numbers of each element in one molecule of hydrogen peroxide. The molecular formula is always a whole number ratio of the empirical formula. For some compounds the molecular formula and the empirical formula are the same, an example would be water H2O. Structural formulas use lines to represent covalent bonds and shows how atoms in a molecule are connected. The structural formula for hydrogen peroxide is H-O-O-H .The number of lines between each element indicate the number of shared pairs of electrons between the two. All bonds in hydrogen peroxide are single lines and indicate one shared pair of electrons. It is possible to also have a double bond (O=C=O) and a triple bond (NΞN). We will discuss the reason behind these types of bonds in more depth a little later in the course, for now you just need to know what they represent. 1. List and explain the different ways to represent compounds. 1|Page 2. What is the difference between an empirical formula and a molecular formula? 3. Write the correct molecular formula for a compound that contains one oxygen atom and two lithium atoms. 4. Determine the number of each type of atom in each formula a. BaCl2 ______________________ _____________________ b. Fe(NO2)2 _____________________ ______________________ _____________________ c. 2Ca(OH)2 ____________________ ______________________ ______________________ 5. Write the empirical formulas for the compounds represented by the following molecular formulas. a. C4H8 ____________________ b. B2H6 _______________________________ c. CCl4 ____________________ d. C5H12 ____________________ e. Hg2Cl2 ____________________ f. C2H4O2 ____________________ 6. Write the empirical formula and molecular formula for the following molecule. H-CΞC-H Empirical formula Molecular Formula ______________ ______________ for class discussion 2|Page