Art Integrated Science Lesson
advertisement

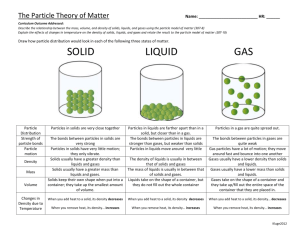
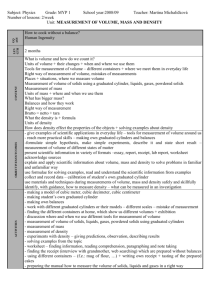
Mary Keck 6 May 2014 Final Exam Science SOL 5.4-Matter I. Purpose a. The purpose of this lesson is to help students understand, on a molecular level, the differences between solids, liquids, and gases; and their physical properties. b. Science SOL 5.4-Matter: The student will investigate and understand that matter is anything that has mass, takes up space, and occurs as a solid, liquid, or gas. Key concepts include: i. Atoms, elements, molecules, and compounds ii. Mixtures including solutions; and iii. The effect of heat on states of matter II. Objectives a. The student will be able to identify solids, liquids, and gases in everyday life; as well as understand and be able to explain the differences between them. III. Procedure: a. Introduction: i. The teacher will begin by introducing solids, liquids, and gases that students interact with on a daily basis. ii. Then, the teacher will ask the students to say what the physical differences are between solids, liquids, and gases (solids are hard, you can’t see gases, liquids are wet, etc.), and write these differences on the board as the students name physical characteristics of each. b. Development i. The teacher will explain the differences of solids, liquids, and gases, at the most basic level: the distance between the atoms. For solids, the atoms are very very close together, which is why solids are hard. For liquids, the atoms are close but not touching, which is why we can put our hands through water. And for gases, the atoms are very far apart, which is why we can’t see them. The teacher will illustrate this on the chalk board above the listed physical characteristics earlier listed. ii. Students will create a “Solid, Liquid, Or Gas?” chart in which they use cheerios to illustrate how solids, liquids, and gases look at a molecular level. For solids, the cheerios will be very close together. For liquids, they’ll be a little farther apart. And only two or three cheerios will be in the gases square. iii. Then, they will write a description of what each a solid, a liquid, and a gas is, including physical properties. iv. Lastly, they will name and draw examples of solids, liquids, and gases. c. Summary i. The teacher will conclude the lesson by reviewing the differences between solids, liquids, and gases; and going over the illustrations/lists on the chalkboard. Then the students will go around and share what their examples for each were. IV. Materials and resources needed for the lesson a. Paper, markers, cheerios, glue—really cheap (and great) lesson! V. Assessment a. The teacher will informally assess each student’s understanding by looking at their charts, and listening to the examples they share at the conclusion of the lesson. If some students still seem “iffy” on the subject matter, the teacher can formally assess understanding through a quiz which asks the students to illustrate the atoms, name physical properties, and give two examples of solids, liquids, and gases.