Lecture 8
advertisement

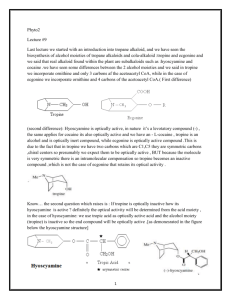
Lecture 8 Today we will continue discussing the physicochemical properties of alkaloids. Generally we said that alkaloids are crystalline solids, and we preferred these types of solids because they can be easily purified by crystallization ,but nevertheless we still have alkaloids that are occurring in an amorphous state , moreover we have alkaloids which are lacking oxygen in there structure so occurring in a liquid (volatile or non-volatile) state and alkaloids which contain oxygen in their structure are in the solid state, with regard to the color most o them are white, but still it depends if we have conjugated system or poly alcoholic one then expected to have yellow, orange up to red. Now , Solubility Depending on if the alkaloids occurring in the form of free bases or their salts, free bases are soluble in non-polar solvents (organic solvents) while their salts are soluble in polar solvents including water, but still it’s a General Statement because we have free bases that are soluble in water but not in an organic solvents like (Caffeine,Ephedrine,Codeine,Colchicines and Quaternary amine) at the same time we have salts that are soluble in organic solvents. Isomerization It happens because of the occurrence of the chiral center either in D form, L form or racimec mixture of both D and L, this isn’t a very specific measurement for the activity of the alkaloids we have alkaloids that are active in the L form others in the D form. Chemical Properties Alkaloids classified into Primary, Secondary, Tertiary amines, Quaternary ammonium salts. Basicity depending on this classification : The most basic is the Primary amine and the least basic is the Quaternary amine. Saturated hexacyclic amines are more basic than aromatic amines. Bases can be classified into: Weak Strong Amphoteric Neutral Phenolic Alkaloids Alkaloids with Carboxylic Acid Caffeine Atropin Psychotrine Cholchicine Morphine Narceine (is a narcotic in opium) Depending on previous classification we can determine the type of acid that can be used to form the bases salts. Stability Generally salts are more stable than their free bases and this is a general observation that most of the secondary metabolites (salts) are more stable than their aglycons. And in the presence of heat the alkaloids some of them not all of them definitely are deteriorated or some of the alkaloids Sublime ) (يتسامىi.e caffeine. Caffeine at high temperatures will sublime at the inner face of the coffee production machine to form caffeine crystals; we scrub these crystals to obtain the pure caffeine. Also with the acids again free bases will form salts, acids involved also in the hydrolysis process, i.e esters are catbolized into acid moiety and alcohol moiety (i.e. Atropine), concentrated acid should not be used because concentrated acid can cause elimination of one molecule of water so the alkaloids will be converted to their apo-derivative, and the prefix apo indicates dehydration process. OR concentrated acid will cause the elimination of Methoxy group. So Dehydration or Demethxilation can be easily achieved in the presence of concentrated acid. Alkalies: Like acid also alkaline may affect stability of alkaloids, when we add diluted ammonia we can convert alkaloids salts to there free bases, sometimes they may cause Isomerization or Racimization (convert D to L or vice versa). Diluted alkaline can form salts from alkaloid containing carboxyl groups. Strong alkalis such as NaOH or KOH form salts with secondary metabolites containing Phenolic Alkaloids to be converted into their corresponding Sodium Phenolate or Potassium Phenolate.(salts) Strong alkalis like diluted acid may hydrolyze esters into their acid and alcohol moieties in addition they affect also amide alkaloids like Colchicine the amide group on the side chain can be splitted. Strong alkali causes opening of lactone ring and this can be used for the identification of the presence of the lactone ring because when it’s opened it will give fluorescence like in coumarine when we ad KOH it will give fantastic fluorescence. Light and oxygen In the presence of light and oxygen alkaloids are mostly unstable and this effect may appear obviously by changing the color from colorless to red, some compounds may decompose, we may have to do certain tests to detect the effect of light and oxygen on specific alkaloids which may be converted from active into inactive form but this may not visible so we have to test for the activity. There are different tests to determine if there’s alkaloid, basically we have precipitating and coloring reagents, keep in mind that we never depend on one positive test; usually we should apply 3-4 different color reactions and 1-2 precipitation reactions (i.e. not every orange color is for alkaloids). There are several other compounds that may give results for false negative or false positive reactions, when we are using the coloring and the precipitating reagents basically we use them just to screen if the plant or any material we are using containing alkaloids or not to do our isolation procedures then we reapply these tests to see if our extraction process was complete and to see how successful our work is, (i.e if we finish the extraction procedure depending on a positive test for the alkaloids at the beginning and when re-administrate other tests on the extract we detect negative results, this illustrates that there are other compounds in the extract so we have to re-repeat the isolation process). Now, Coloring Reagents: most of them containing concentrated acid that may cause oxidation, dehydration or opening of the lactone ring and they are mostly destructive reagents. We have first type of General coloring or precipitating reagents and second type of Speciefic reagents, specific reagents can be used for certain groups for Ex; Tropane alkaloids. We have Very Specific Tests that give positive results only for one single compound for Ex; detecting Quinine in Cinchona. To sum up we have: 1- General Tests. 2- Specific Tests. 3- Very Specific Tests. Using any reagents depends on the procedure we are following or the objective we are looking for. Definitely after isolation we have primary and secondary metabolites starting with melting point determination and optical rotation (UV, IR, NMR, MS ). Alkaloids have nitrogenous secondary metabolites and very different basic chemical structures (Phenylalkylamines, Pyridine, Piperidine, Tropane , Quinoline , Isoquinoline, Imidazoline , Steroidal and Terpenoidother heterogeneous basic nuclei that will be discussed later) Terpenoid have N in their structure but they aren’t true alkaloids because it’s not a derivative of an amino acid and the N introduced to its structure by a Transamination Reaction. Vast majority of the alkaloids are originated from 5 amino acids 2 aliphatic (Orinthine, Lysine) and 3 aromatic amino acids (Phenylalanine, Tyrosine and Tryptophan) BUT there are other amino acids involved in the synthesis of alkaloids. Alkaloids derived from L-Orinthine: Orinthine is non-protein amino acid that posses two amino groups one is alpha (α) the other is Delta (δ) provides C4N building block to the alkaloids; found with Pyrrolizidine, Indolizidine, Nicotiana and Tropane alkaloids . ــــCO2 Oxid. DA Refer to slides to see the structure mentioned here. Figure (1) Figure (2) And even starting with ornithine to end up with Pyrrolidine there are more than one possibility this is depends on organism alternative pathways, in the simple case de-carboxylation to get pyogenic amine then the carboxylic group is eliminated to get amine, because these substances are biologically active, the next step is cyclization via intermediate to get the heterocyclic pyrrolidine. We can go via a Tracin and end up with pyrrolidine, different amines they don’t posses a pleasant smell. What is the meaning of Tracin: it’s a deteriorated animal meat, on the other hand when making an ester you will smell a very nice smell. In Orinthine we have 2 amino groups now which one is used and which one is deleted, nowadays it’s easy to know by using radioactive paper and it has been found that alpha amino group is the one to be lost, delta amino group will be used for synthesis our compound and this is more clearly demonstrated not via pyogenic amine (decarboxylation) but via transamination reaction to get alpha keto delta amino ethanoiec acid this is a pentanoic acid (5 carbons) in which the amino group is changed into keton group via simple transamination or oxidative deamination reaction to get alpha keto delta gamma delta amino pentanoic acid which via intermediates will be cyclized in to pyrrolidine. In both amino acid Orinthine and Lysine we have two amino groups, using amino acid Lysine we get piperidine. Lysine also decaboxylated into pyogenic amine (Cadaverine) from Cadaver which means very bad smell compound and this is going to be cyclized into Piperidine ring, or by oxidative deamination (transamination) pathway Alpha Keto Beta Gamma Delta hexanoic acid (6 carbons) will be converted to Alpha Keto Exelon amino hexanoic acid (cabroic acid) then be cyclyezed into piperidine. >>>>Refer to the slides<<<< alkaloids derived from L-Lysine So both amino acids lose their alpha amino groups and use either Delta (orinthine) or Exelon (Lysine) amino groups for the formation of heterocyclic rings. We squeeze here lysine to show the similarity between these two amino acids, again orinthine derivatives are pyrrolidine and tropane. Tropane Alkaloids Now, we will started with biggest derivative of L-Orinthine which is Tropane Alkaloids, we use Atropine which it’s source is the nature because synthesis isn’t economically feasible comparing to the isolation from the nature, tropane alkaloids not wildly distributed Tropane alkaloids are developed from the five member ring Pyrrolidine, they are occuring in Solanaceae , Erethroxylaceae (it’s small but it’s important because cocaine is isolated from it) , Convolvulaceae. It’s the parent base of several alkaloids (bi-cyclic) when we recognize the structure of tropane we can see five member heterocyclic ring plus six member heterocyclic ring (Pyrrolidine, Piperidine) . see figure (2) above. Tropane Ring Figure (3): Atropine This is a question in the exam: where amino acid Lysine is included >>> giving several structures including Tropane >>>>>>>> Lysine NOT included in Tropane . Tropane alkaloids are derivative of the amino acid L-orinthine although we have Piperidine in it’s structure and we said that Piperidine developed from the amino acid lysine >>>>>> which means that there are other possibilities in the nature to develop Piperdine other than from Lysine. Tropane alkaloids they are occurring in form of their ester, so it has hydroxyl group (alcohol) and the origin of this (OH) is from the biosynthesis. By numbering the Tropane structure , positions 1 and 5 they are linked with N, we have alcohol moiety at position # 3 (Tropan-3α-ol) it’s isomeric it can occur in alpha (OH) or beta (OH), so we will have two types of alcohol >>> Tropan-3α-ol (Tropine) it’s the preferred alcohol moiety from the family Solanaceae + Erethroxylaceae and Tropan-3β-ol (Psuedotropine) Figure (4) In the nature in the alkaloids chemistry we have major and minor alkaloids, in the families Solanaceae + Erethroxylaceae our alcohol is minor compound, other derivatives of pyrrolidine they are major (tiny) and not only found in these families but in others too, the less complicated structure the most distributed in the nature, the more complicated structure the more restricted for some plant families and genera or even species. Figure (5) If we look to the simple structure tiny compound Hygrine it’s pyrrolidine to which an acetyl group attached to the N alpha position, this hygrine or it’s dimer Phosphahygrine are minor derivative (not primary) formed by the adding of AcetylCOA to pyrrolidine then reduction then elimination of methyl group to have hygrine then another AcetylCOA is added, decarboxylation and another pyrrolidine we will obtain phosphahygrine,they are minor alkaloids in Solanaceae + Erethroxylaceae and also found in other plant families. (Written as doctor said!!!!) <<<<<Paragraph talking about Hygrine from the book, read it for further understanding>>>>> Figure (6) Return back to Tropane structure >>>> Figure (4) Now we will see how we will synthesize our alcohol, we said that OH group is positioned on C3 BUT we now we will know the origin of this OH. We started with L-orinthine the pyogenic amine and end up with pyrrolidine, in between we have Pyrrolinium cation as an intermediate which is a precursor, Pyrrolinium before coming intact to form terminal compound pyrrolidine a condensation of AcetoacetylCOA with Pyrrolinium cation yields an intermediate after cyclization will end up with the formation of Tropanon. because we have C=O group and C.Acid so it’s Tropanone carboxylic acid obtained by the condensation of cyclic derivative of ornithine with AcetoacetylCOA, now we have C=O group on position #3, a reduction reaction to have alpha or beta OH, but there’s a carboxyl group from AcetoacetylCOA this carboxyl group will be de-carboxylated to get Tropinone which will be reduced into Tropine and Psuedotropine . In majority of cases Tropine (Tropan-3α-ol) reduced (in Solanaceae) and esterification happens we get Tropic acid or any other acid we have different possibilities. Tropine is obtained from >>>>>> ornithine and 3 Carbons from AcetoacetylCOA. But if No de-carboxylation and No reduction happens, so we keep orinthine and 4 carbons from AcetoacetylCoA at this stage we form Coca Alkaloids (Cocaine) they are esters (di-esters) because we have carboxyl group to which we can add alcohol to form an ester and hydroxyl group to which we add an acid and form an ester. >>>>> In cocaine and related substances we keep orinthine and 4 cabons from AcetoacetylCoA and sense we have in the same molecule a carboxyl and a hydroxyl group so we have di-ester. In cocaine we add methanol, a methyl ester using benzoic acid. With radioactive experiments it has been found that the carbons 2 and 4 in Tropane or Tropine of cocaine are from AcetoacetylCoA . >>>>>>>> Refer to the last Figure in the sheet. Figure (7): Tropane Note: Very Important To Return Back To The Doctor’s Slides. Done By: Heba Jabr Good Luck