Materials List and Review Request - Livestock
advertisement
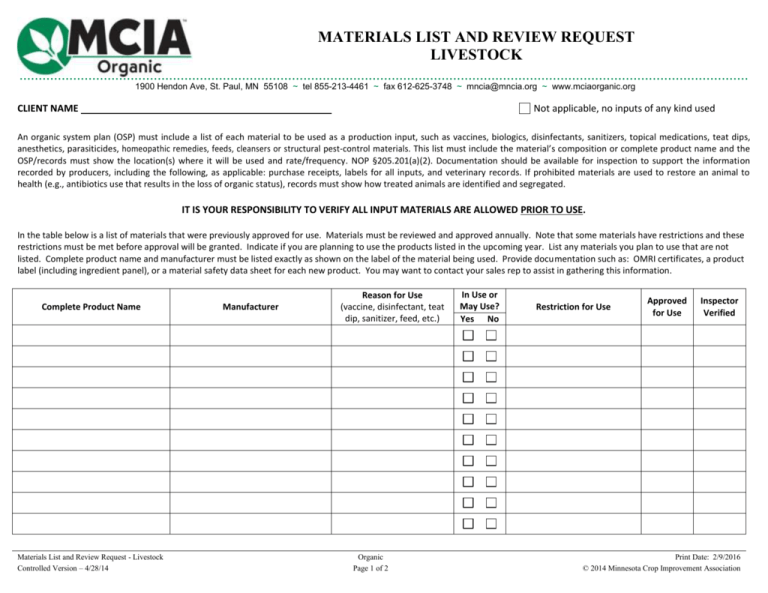
MATERIALS LIST AND REVIEW REQUEST LIVESTOCK .............................................................................................................................................................................................. 1900 Hendon Ave, St. Paul, MN 55108 ~ tel 855-213-4461 ~ fax 612-625-3748 ~ mncia@mncia.org ~ www.mciaorganic.org CLIENT NAME Not applicable, no inputs of any kind used An organic system plan (OSP) must include a list of each material to be used as a production input, such as vaccines, biologics, disinfectants, sanitizers, topical medications, teat dips, anesthetics, parasiticides, homeopathic remedies, feeds, cleansers or structural pest-control materials. This list must include the material’s composition or complete product name and the OSP/records must show the location(s) where it will be used and rate/frequency. NOP §205.201(a)(2). Documentation should be available for inspection to support the information recorded by producers, including the following, as applicable: purchase receipts, labels for all inputs, and veterinary records. If prohibited materials are used to restore an animal to health (e.g., antibiotics use that results in the loss of organic status), records must show how treated animals are identified and segregated. IT IS YOUR RESPONSIBILITY TO VERIFY ALL INPUT MATERIALS ARE ALLOWED PRIOR TO USE. In the table below is a list of materials that were previously approved for use. Materials must be reviewed and approved annually. Note that some materials have restrictions and these restrictions must be met before approval will be granted. Indicate if you are planning to use the products listed in the upcoming year. List any materials you plan to use that are not listed. Complete product name and manufacturer must be listed exactly as shown on the label of the material being used. Provide documentation such as: OMRI certificates, a product label (including ingredient panel), or a material safety data sheet for each new product. You may want to contact your sales rep to assist in gathering this information. Complete Product Name Materials List and Review Request - Livestock Controlled Version – 4/28/14 Manufacturer Reason for Use (vaccine, disinfectant, teat dip, sanitizer, feed, etc.) Organic Page 1 of 2 In Use or May Use? Yes No Restriction for Use Approved for Use Inspector Verified Print Date: 2/9/2016 © 2014 Minnesota Crop Improvement Association CLIENT NAME Complete Product Name Manufacturer Reason for Use (vaccine, disinfectant, teat dip, sanitizer, feed, etc.) In Use or May Use? Yes No Restriction for Use Approved for Use Inspector Verified USE OF ALL MATERIALS MUST BE NOTED IN YOUR RECORDS. Approval of materials does not allow or encourage the commercial use of MCIA’s name or seal in association with these materials, nor does it imply endorsement of the material or manufacturer. Materials List and Review Request - Livestock Controlled Version – 3/27/2014 Organic Page 2 of 2 Print Date: 2/9/2016 © 2014 Minnesota Crop Improvement Association











