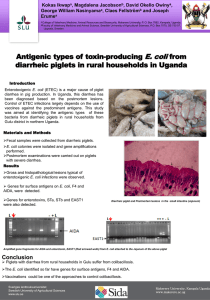
Bacterial Contamination of Drinking Water in Rural
advertisement

BACTERIAL CONTAMINATION OF DRINKING WATER IN RURAL GHANA Katherine Tracy Parker B.S., Harvey Mudd College, 2000 THESIS Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in BIOLOGICAL SCIENCES at CALIFORNIA STATE UNIVERSITY, SACRAMENTO FALL 2011 BACTERIAL CONTAMINATION OF DRINKING WATER IN RURAL GHANA A Thesis by Katherine Tracy Parker Approved by: __________________________________, Committee Chair Robert Metcalf, Ph.D. __________________________________, Second Reader Susanne Lindgren, Ph.D. __________________________________, Third Reader Enid Gonzalez, Ph.D. ___________________________ Date ii Student: Katherine Tracy Parker I certify that this student has met the requirements for format contained in the University format manual, and that this thesis is suitable for shelving in the Library and credit is to be awarded for the thesis. _________________________, Graduate Coordinator Susanne Lindgren, Ph.D. Department of Biological Sciences iii _________________ Date Abstract of BACTERIAL CONTAMINATION OF DRINKING WATER IN RURAL GHANA by Katherine Tracy Parker The disease burden from the lack of biologically safe water to drink has been observed in Ghana, where shallow hand-dug wells, deep-drilled boreholes, and other point sources constitute the rural drinking water supply. The low-cost, easy-to-use “Portable Microbiology Laboratory,” containing both a Colilert field test for the presence or absence of E. coli, as an indicator of fecal contamination, and a Petrifilm field test to enumerate E. coli colony forming units, was used to classify the disease risk of 146 rural drinking water point sources and 21 household water storage units in two districts of Ghana from "low" to "very high," in accordance with World Health Organization standards. The results showed that drinking water from 85% of boreholes was low risk of disease, while only 6% of water from hand-dug wells and other surface point sources was low risk of disease. This verified the strategy of the Ghana government of constructing boreholes to improve the safety of drinking water for the rural population. Use of the Portable Microbiology Laboratory to test water stored in homes, which had been obtained from the nearby borehole, showed generally higher disease risk in the home-stored water than in the water directly from the borehole. The results also demonstrated variations in iv the disease risk by region and changes in the disease risk for particular point sources over a relatively short period of time. This study demonstrated the effectiveness of the water tests in the Portable Microbiology Laboratory in rural Ghana to rapidly identify drinking water sources that had a high risk of disease. ___________________________, Committee Chair Robert Metcalf, Ph.D. ___________________________ Date v ACKNOWLEDGMENTS Many thanks to Gabriel M. Nartey, Gershon Hiamadey, and the members of the Suhum District Water and Sanitation Team who provided transport via governmentissued motorbike, translation, community connections, and valuable insights. Their support made testing in the Suhum District possible. Similarly, testing in the Nanumba District was only able to occur because of the transport, translation, community connections, and commitment to community improvement of Abu Mutaru Abass, who served as an Area Coordinator for Guinea Worm Eradication. Theo Mensa, who is an engineer for the Eastern Region Water and Sanitation Team, provided access and information that substantially furthered my understanding, as did Charlotte Engmann, an engineer working out of CWSA headquarters. Hospital personnel graciously provided, and I much appreciated, the Monthly Out-Patient Morbidity Returns for the Suhum-Kraboa-Coaltar District Hospital in the Eastern Region of Ghana, for the period of January to December 2005 and January to September 2006. Joseph Ampofo generously shared his experience in water testing in Ghana and in the current practices and procedures of the country's national laboratory for biological water quality assessment. vi The faculty and staff at the University of Ghana, Legon were dedicated to providing a rich learning environment. Special thanks to Irene Odeti, who expertly facilitated my presence in Ghana in an exchange between California State University, Sacramento and the University of Ghana, Legon. Professor F.K.E. Nunoo in the Department of Oceanography and Fisheries provided expert advice and guidance for this research effort while I was in Ghana and gave helpful comments on early drafts. vii TABLE OF CONTENTS Acknowledgments.............................................................................................................. vi List of Tables ...................................................................................................................... x List of Figures .................................................................................................................... xi INTRODUCTION .............................................................................................................. 1 Context of the Study ....................................................................................................... 1 Water Surveillance .......................................................................................................... 2 E. coli Direct Testing as an Indicator of Water Quality ................................................. 7 Water Safety Plan ......................................................................................................... 13 Objectives ..................................................................................................................... 15 MATERIALS AND METHODS ...................................................................................... 18 Study Sites and Sample Collection ............................................................................... 18 Colilert Defined Substrate Medium (10 ml test)........................................................... 19 E. coli Count Petrifilm (1.0 ml test) .............................................................................. 21 Visual Sanitation Survey of Boreholes ......................................................................... 22 Statistical Analysis ........................................................................................................ 22 Quality Assurance ......................................................................................................... 23 RESULTS ......................................................................................................................... 25 Contamination Levels in Hand-dug Wells and Other Shallow Sources ....................... 27 Contamination Levels in Boreholes .............................................................................. 33 Visual Sanitation Survey of Boreholes ......................................................................... 39 viii Novel Testing Method for 100 ml Samples .................................................................. 39 Independence of Well Sources Within a Community................................................... 42 Comparison of Boreholes and Hand-dug Wells ........................................................... 42 Comparison of Disease Risk Across Geographical Variation ...................................... 45 Comparison of Disease Risk Across Seasonal Difference ........................................... 45 Contamination Levels in Household Water Storage Units ........................................... 48 DISCUSSION ................................................................................................................... 53 Point Sources ................................................................................................................ 55 Comparisons ................................................................................................................. 61 Application of Findings ................................................................................................ 72 APPENDICES .................................................................................................................. 80 Appendix A. Management of the Rural Water Supply in Ghana .................................... 81 Appendix B. Water Facilities Monitoring Sheet for WatSans .......................................... 86 Appendix C. Survey of Point-Source Water Supply ........................................................ 89 Literature Cited ................................................................................................................. 91 ix LIST OF TABLES Table 1. Determination of WHO disease-risk categories for drinking water and correlation of Colilert and E. coli count Petrifilm results with risk categories. ....................................................................................................... 11 Table 2. Test results for drinking water from shallow, hand-dug wells. ..................... 30 Table 3. Test results for drinking water from open water sources............................... 32 Table 4. Test results from boreholes in the Suhum District during the short rainy season (October). ............................................................................................ 35 Table 5. Test results for boreholes in the Nanumba District during both the dry (March) and rainy (June) seasons. .................................................................. 36 Table 6. Comparison of disease-risk level of water sources within and between communities. .................................................................................................. 43 Table 7. Comparison of WHO disease-risk levels in paired water test results for boreholes in the Nanumba District during two seasons, dry and rainy. ......... 47 Table 8. Water test results from household storage units in the Nanumba District, rainy season. ................................................................................................... 50 Table 9. Order of magnitude change comparison of contamination levels in households to the contamination level at the nearest borehole. ..................... 52 x LIST OF FIGURES Figure 1. Using the Portable Microbiology Laboratory in Ghana. ................................... 26 Figure 2. Surface and shallow water sources at each WHO disease-risk level in the Suhum District. .................................................................................................. 31 Figure 3. Comparison of boreholes by region and season at each WHO disease-risk level. ................................................................................................................... 38 Figure 4. Comparison of boreholes at each WHO disease-risk level for various score ranges on the sanitation survey. ......................................................................... 40 Figure 5. Comparison of Colilert test results from 10 ml and 100 ml borehole samples. 41 Figure 6. Comparison of boreholes and hand-dug wells in the Suhum District at each WHO disease-risk level. .................................................................................... 44 Figure 7. Comparison of boreholes at each WHO disease-risk level in the Nanumba District during two seasons, rainy and dry......................................................... 46 Figure 8. Comparison of closest boreholes and household drinking supplies in Nanumba District (rainy season) at each WHO disease-risk level. ................... 51 Figure 9. A University of Ghana teaching assistant talks to a group of school children about the importance of proper well maintenance. ............................................ 71 Figure 10. The District Water and Sanitation Team can simultaneously take a variety of measurements and perform maintenance on a borehole. ............................... 75 xi 1 INTRODUCTION Context of the Study The international community well recognizes the importance to public health of sufficient quantities of safe drinking water. The United Nations emphasizes the importance in declaring 2005-2015 the International Decade for Action: Water for Life. Similarly, Target 7.C of the Millennium Development Goals of the United Nations is to “halve, by 2015, the proportion of the population without sustainable access to safe drinking water and basic sanitation.” Worldwide, approximately 2.6 billion people do not have access to improved sanitation and approximately 900 million people are without improved drinking water sources (40). The lack of safe water contributes to the approximately 4 billion cases of diarrhea and about 1.8 million deaths every year in developing countries (40). Of these deaths, 90% are of children under five, which accounts for about 19% of total child deaths (4, 32). The global disease burden from diarrhea in developing countries has also been observed in Ghana, where this study is focused (Suhum-Kraboa-Coaltar (Suhum) District Hospital, personal communication). For rural and small-town communities in Ghana, the responsibility for promoting and sustaining a safe water supply is delegated to the Community Water and Sanitation Agency (CWSA). The CWSA coordinates the construction of community-owned and managed sources of drinking water in rural areas. (For further background, see Appendix A.) These are “point sources” and primarily take the form of boreholes, which are deep- 2 drilled wells (50 to 120 m) with an attached hand-pump. Some communities also have alternative point sources available, such as shallow, cement-lined, hand-dug wells (10 to 30 m, with or without a pump), rainwater collection systems, and ponds that are seasonal or year-round. The testing of biological water quality in such point sources and how that testing relates to the public safety policy of CWSA to drill boreholes in replacement of alternative sources of rural drinking water is the context of the study addressed by this paper. Water Surveillance Since the 1880s when cholera, typhoid and bacterial dysentery (shigella) were demonstrated to be caused by pathogenic bacteria, microbiological quality has been recognized to be one of the characteristics that is important to a community’s drinking water supply. In addition to the microbiological quality, indicators that are important in the surveillance of water to ensure that it meets the needs of the community include: (1) per person daily quantity used, (2) the reliability of the supply, (3) the cost, and (4) the percentage of the population with access (16). The CWSA periodically, though infrequently, studies water usage and collects information on all of these indicators except for microbiological quality. “District Water and Sanitation Teams” in the CWSA Eastern Region, for example, as part of their management responsibilities in partnership with village-level water and sanitation committees (WatSans), use a “Water Facilities Monitoring Sheet for WatSans” to collect data on indicators one through four for boreholes and hand-dug wells with hand-pumps and rainwater catchments (Appendix B). 3 The CWSA data collection for microbiological water quality in Ghana, however, is limited by the availability of laboratory facilities that can perform traditional monitoring tests and by the cost and time involved in transporting samples. These are major limitations, which lead to few tests and long intervals of time between microbiological testing. This is an unfortunate circumstance for the health of rural communities in Ghana insofar as reducing the risk of waterborne diseases. Studies have found that the microbiological quality of water can vary significantly over short periods of time and therefore water should be monitored frequently to ensure low disease risk to the community using the supply (1, 16). Concern about Fecal Contamination of Drinking Water. Microbiological pathogens that are transmitted by the fecal-oral route, especially those originating from human feces, are of particular concern for water quality surveillance programs focused on public health. Fecal-oral pathogens can be transmitted from the excreta to the mouth via water, flies, hands, or food (16). Bacteria that cause fecal-oral infections include Campylobacter jejuni (dysentery), Escherichia coli (diarrheal infection or dysentery), Shigella spp. (dysentery), Salmonella spp. (acute diarrheal infection), Salmonella typhi (typhoid fever), Yersina enterocolitica and Y. pseudotuberculosis (acute diarrheal infection), and Vibrio cholerea (cholera). Studies have shown that diarrheal disease in developing countries can be reduced in occurrence by 20% through increased water availability, which provides the opportunity for increased effective hand washing (32). Furthermore, improved drinkingwater quality can lead to a reduction in occurrence of adult diarrhea by 15% and up to a 4 40% reduction for infant diarrhea when provided in conjunction with appropriate sewage disposal (32). In addition to bacterial pathogens, viruses transmitted in fecally-contaminated water are well recognized as agents of diarrheal disease and mortality (16). Of particular concern for children is Rotavirus, which is the number one cause of diarrheal disease and infant mortality in developing countries today. Viruses such as Hepatitis A and E (fever and jaundice) and Norwalk agent are also spread by fecal contaminated water. Several protozoa are the cause of fecal-oral infections, including Entamoeba histolytica (protozoal diarrhea), Giardia lamblia (protozoal diarrhea), and Cryptosporidium parvum (protozoal diarrhea). Water Testing Policy in Ghana. Testing drinking water for biological quality is essential in providing information about disease risk and when disinfection methods are needed to protect public health. What, though, is the optimal frequency of such testing for the protection of public health? The CWSA policy at the time of this study only recommended the microbial testing of two boreholes per district every year. In the Suhum District of Ghana's Eastern Region, for example, there are 181 boreholes located in 98 communities. The CWSA policy results in testing only one percent of the Suhum District sources each year, a frequency that presents three questions of adequacy: (1) Will testing a well once per year ensure the quality of the water for the entirety of the year? (2) Will testing only 1% of the water point sources in a district ensure the quality of all the sources in the district? (3) Will testing only two sources give a sufficient picture of the situation in the district to validate that the policy is effective in meeting its goals? 5 More extensive testing, it would appear, is not a CWSA priority for public health. Why? A major reason water quality testing is sometimes given lower priority in public health discussions in developing countries (far behind HIV/AIDS, malaria, and tuberculosis discussions, e.g.), is the perception of limited availability of modern and efficient water testing procedures. Unfortunately, the procedures and equipment most widely known to be available, as discussed further below, are obsolete or cumbersome. History and Development of Water Testing. The general importance of water quality and testing to insure good water quality has long been established. In the 1880s, typhoid fever, cholera, and bacterial dysentery (Shigella sp.) were discovered to be caused by bacteria entering water from fecal contamination, which lead to a general recognition of the importance of water testing to public health. Because testing directly for all pathogens is impractical, microbiologists sought a universal microbial indicator of fecal pollution and identified Escherichia coli (E.coli) as the best indicator (10, 12, 14, 35). E. coli were acknowledged as the best indicator of recent fecal contamination because it is universally present in large numbers in the feces of humans and warmblooded animals; it does not multiply once it leaves the body and enters water; it survives in water and is removed from water in a manner similar to waterborne pathogens; and it is relatively easy to detect (10, 12, 14, 35). Thus, the WHO established that the presence of E. coli provides conclusive evidence of recent fecal pollution and therefore E. coli should not be present in drinking water (39). 6 Measurement of Indicator Bacteria. There are two general types of bacteriological assessment of water quality, quantitative and qualitative. In the quantitative method, the number of bacteria is expressed as colony forming units (cfu) per volume of water. This term comes from counting the number of colonies that grow either on a membrane filter or in a Petri plate. The qualitative option is to report the presence or absence (P/A) in a set volume of water, typically 10 ml or 100 ml. A P/A test can also be used to estimate the microbial concentration (cfu per volume) using multiple and varying sample volumes (typically nine tubes: three dilutions in triplicate). This is a maximum likelihood analysis method and is reported as a Most Probable Number (MPN). Thermotolerant Coliform Test. A century ago, there was no simple test specifically to identify E. coli. Substitute tests were developed based on the ability of E. coli to produce gas from lactose at 44-44.5°C. This test was first called the fecal coliform test. (Although E. coli is a coliform bacterium, the term “coliform” as used in this paper will refer to coliforms other than E. coli.) The fecal coliform test was later renamed the thermotolerant coliform test, as some coliform bacteria besides E. coli, particularly members of the genus Klebsiella, may also produce gas from lactose at this elevated temperature. Klebsiella grow in the environment and are not indicators of fecal pollution. Because of this ambiguity in interpretation of test results, the thermotolerant test is not considered today to be a definitive measure for the presence or absence of E. coli without follow-up confirmation (12, 22, 35). WHO regards the thermotolerant test as a less 7 reliable, but acceptable, index of fecal pollution when specific testing for E. coli is not performed (39). In the rare occasions when water testing is performed in Ghana, and in most other developing countries, the thermotolerant test is performed. This requires an autoclave to sterilize the lactose containing tubes used in the test, incubators at 35°C and 44° or 44.5°C, and trained personnel. Where field-testing kits are available, such as Oxfam’s Del Agua unit, Wagtech Potatest, or the ELE Paqualab, they are expensive, bulky and cumbersome. They can be transported by car or truck, but not by motorbike. Not only do they require extensive media preparation and in-field disinfection supplies, they also require battery power to run the incubator, which is not available in most rural areas of Ghana. E. coli Direct Testing as an Indicator of Water Quality In the 1980s, advances in the understanding of genomic type bacteria of the family Enterobacteriacia led to the discovery that E. coli, but not other coliforms, contained the constitutive enzyme β-glucuronidase. This discovery enabled direct testing for E. coli in water, which makes the inexact coliform and thermotolerant coliform tests obsolete. The new tests, including Colilert and Petrifilm, discussed below, detect and enumerate E. coli easily, rapidly, cheaply, and independently of an equipped laboratory (1, 7, 11, 13, 15). Because of these advances, neither coliforms nor thermotolerant coliforms, the older targets of testing, are now considered to be acceptable substitute indicators for E. coli (22). 8 P/A Testing for E. coli with Colilert. In 1987, IDEXX Laboratories, Inc. (Westbrook, Maine) introduced the Colilert test, where MUG (4-methyl-umbelliferyl-βD-glucuronide) is the substrate for glucuronidase and is hydrolyzed to the fluorescent 4methyl-umbelliferone (MU) and glucuronide, which E. coli uses as an energy source for growth (12). The Colilert test also contains the substrate ONPG (o-nitrophenyl β-Dgalactopyranoside), for the β-galactosidase enzyme present in all coliform bacteria. This is hydrolyzed to release the yellow o-nitrophenyl (ONP). Environmental coliforms will only turn the Colilert tests yellow, whereas E. coli will also cause a blue fluorescence when a long-wavelength ultra-violet (UV) light shines on the sample. After approval by the U.S. Environmental Protection Agency (EPA) and international organizations, the Colilert test has come to be used in over 90% of all U.S. state labs and more than all other methods combined in the U.S., Canada and Japan drinking water markets (20). In the U.S., a 100 ml Colilert P/A test is most often performed on treated drinking water, as U.S. drinking water standards are based on the absence of any coliform bacterium in 100 ml (42). When the Colilert method was first introduced, it came in a small test tube to which 10 ml of water was added. This was because the EPA standards at that time (1988) were for no coliform in 50 ml of water and inoculation of five 10 ml P/A tubes could provide some information on the extent of coliform contamination in a 50 ml sample. In 1989, when the EPA standard shifted to no coliform in 100 ml, use of the Colilert shifted from the 10 ml P/A to a 100 ml P/A snap-pack of reagents. Fortunately, however, IDEXX still produces the 10 ml P/A test. 9 Enumeration of E. coli with Petrifilm. In the U.S. food industry, the most widely used test to detect E. coli in foods is the E. coli Count Petrifilm, developed by the 3M Company (St. Paul, Minnesota). In this test, approved by the Food and Drug Administration (FDA), a violet-red bile medium contains the glucuronidase substrate BCIG (5-bromo-4chloro-3-indolyl-β D Glucuronide). One ml of a food dilution is added to a circle of dried nutrients, which is then covered with a transparent plastic film and incubated for up to 24 hours at 35°C. If E. coli bacteria are present, a blue colony will develop, as BCIG is hydrolyzed to release the insoluble blue BCI. Gas bubbles also form around the E. coli and other coliform colonies, as lactose in the medium is fermented to acid and gas. Although the E. coli Count Petrifilm was not intended to be used for drinking water in the U.S., because it samples only 1 ml and not 100 ml, it has been effective in detecting E. coli in raw water sources where E. coli concentrations are >1/ml (26, 33, 36). Determination of WHO Categories for Risk of Disease from Water Using the Colilert and Petrifilm Tests. The WHO standard for a very low risk of disease using a P/A test of E. coli in water intended for human consumption is “A” (Absent, zero tolerance) in 100 ml of water. This is also the drinking water standard in developed countries with chlorinated water systems (42). However, because the great majority of the rural water supply, especially in developing countries, is not chlorinated and has widespread occurrence of fecal contamination, other disease-risk categories are also included in the WHO standards: low, moderate, high, and very high (37). WHO suggests 10 that the tolerable risk levels need to be set for each country based on goals to make progressive improvements in the water supply (39). By combining the results of the 10 ml Colilert P/A test with the results of the 1 ml Petrifilm test, four WHO disease-risk categories can be determined based on differences in E. coli concentrations (Table 1). The quantity of 10 ml for the Colilert P/A test is important, as it distinguishes water that has a low disease risk from water that does not have a low disease risk. The quantity of 1 ml tested by the Petrifilm is important to identify high- or very-high-disease-risk water sources. The combination of Colilert and Petrifilm tests cover a four-log difference in E. coli counts, which would be challenging for a single test to cover. These are low, < 1 cfu in 10 ml; moderate, 1-10 cfu in 10 ml; high, 1-10 cfu in 1 ml; and very high, >10 cfu in 1 ml. To be precise, the most favorable category is not designated "safe" or "zero" presence of E. coli or waterborne pathogens, but "low disease risk” (16). 11 Table 1. Determination of WHO disease-risk categories for drinking water and correlation of Colilert and E. coli Count Petrifilm results with risk categories. Disease-risk Level Low Moderate High Very High E. coli in sample < 1 cfu per 10 ml 1 – 10 cfu per 10 ml 1 – 10 cfu per ml > 10 cfu per ml Colilert MUG + + + + # Blue Colonies + gas on Petrifilm 0 0 1 – 10 > 10 12 The Portable Microbiology Laboratory. In order to test water at its source, the Colilert tubes and Petrifilms are combined with sterile plastic pipettes and collecting bags, and a hand-held, long-wave UV light to form a “Portable Microbiology Laboratory” (27, 28). As the tests are stable at room temperature, no special storage conditions are required and materials for 25 of each test can be included in a one-gallon plastic bag. The Portable Microbiology Laboratory is suited to identifying and enumerating both E. coli and coliforms in ranges of concentrations similar to what have been found in other studies in Ghana, without needing to guess at concentrations before sampling (2, 29). For example, a study of three rivers in Ghana, using thermotolerant by membrane filtration, resulted in a range of 66 to 1848 cfu/100 ml (2). Although the low counts could be detected filtering 100 ml, direct testing of water from the most contaminated sources would result in a clogged filter. Dilutions would have to be made so that the amount of source water actually filtered was only 1-5 ml. By using the combination of Colilert and Petrifilm tests, results with a similar level of detail could be obtained with direct testing for E. coli and without guessing about dilutions to filter. Because the Colilert and Petrifilm tests are ready to use with the addition of water, they are practical to use in the field. As E. coli counts need to reach approximately 106 to form a visible blue colony on Petrifilm and about 107/ml to give a MUG positive test in Colilert, a single E. coli cell in these tests would have to double 20-24 times, respectively, to reach these levels. As E. coli grows most rapidly at body temperature of near 35°C, if ambient temperatures are <30°C and results are desired within 12-18 hours, 13 one can incubate Petrifilm and/or Colilert tubes on one's body. In addition, the simplicity of performing these tests and interpreting the results creates the possibility of involving community members in testing their water sources. Water Safety Plan WHO Guidance. The primary aim of the WHO Guidelines for Drinking Water Quality is the protection of public health. In order to implement the guidelines within a national context, WHO also recommends each nation develop a Water Safety Plan and has published guidelines for such a plan that outline a disease-risk management framework (38). The need for, and practice of, water testing in order to monitor effective treatment practices in pipe distribution systems is well established. However, microbiological testing is rarely employed in systems where individual families access water from a point source. In order to meet health-based targets in point source supply areas, the first goal is usually to improve access and quantity of water available, so that all communities meet intermediate access service levels (17). According to the most recent WHO and UNICEF statistics, only 74% of the rural population of Ghana has access to "improved" sources of water that include both boreholes and hand-dug wells (40). In a point-source system, a Water Safety Plan should include “health outcome targets” that are set at the national level. However, “performance targets” of a particular point source that are of concern to the local community are not necessarily within the 14 scope of priorities at the national level, but should be monitored by the community operator (39). The Portable Microbiology Laboratory is a combination of effective tests that is particularly suited to several steps in the development of a Water Safety Plan for point sources. WHO has developed a model water safety plan for monitoring and verifying boreholes fitted with a hand-pump (38). Within the framework of a Water Safety Plan, microbiological testing of point sources can serve three purposes: (1) system assessment as a baseline to set policy, (2) community-based self-monitoring and management of sources, and (3) verification/validation of policy and surveillance (38). Community-based Monitoring. The role of water-quality monitoring within WHO’s model Water Safety Plan for boreholes is relegated to a community operator. In Ghana, this was primarily the role of the WatSan committee, with mobilization and assistance from the CWSA officers. With regard to microbiological testing, the WHO model plan has the community operator check on a monthly basis for leaching of microbial contaminants into the aquifer by verifying that there is no source of fecal material within the setback distance. In the areas of this research, where sanitation facilities were largely non-existent, this would include establishment of a well-marked fecal-free zone. Enforcement of a policy to maintain animals at a safe distance and prevent human free-range defecation within that zone, as well, would then be within the ability of the community operator. Thermotolerant coliform microbiology testing is prohibitively expensive and technologically impossible to do in this context. However, use of the Portable Microbiology Laboratory, in conjunction with regular sanitary 15 inspection, would enable the community operator to monitor the community water sources and to share the results of these tests with the community. The community would then have evidence-based microbiology upon which to self-identify sources of contamination and take corrective action. Suitability for Community-managed Water Supplies. The rural water supply systems in Ghana fall under the classification of “community-managed supplies.” Community-managed water supplies include simple piped systems and a variety of point sources such as boreholes, dug wells and protected springs. This range of water sources poses a problem for surveillance using traditional methods, because the dispersed nature of these small-scale water supplies increases the cost of surveillance. Taking all of the foregoing into consideration, then, and in particular the context of advances in water testing technology and concern about public health in the developing world relative to biologically safe drinking water, and being mindful of WHO guidance and the governmental infrastructure in Ghana, this author developed some hypotheses for study. Objectives I hypothesize that the Ghana Community Water and Sanitation Agency (CWSA) is meeting its mandate of providing low-disease-risk drinking water (defined here as a tolerable disease-risk level of no E. coli in 10 ml of water) through the construction of boreholes. I have five subsidiary hypotheses. 16 (1) There will be a lower level of E. coli contamination in boreholes than in handdug wells and open sources. (2) There will be a lower level of E. coli contamination in boreholes during the dry season than during the wet season. (3) There will be a lower level of E. coli contamination in boreholes in the Northern, arid region (Nanumba District) than in the Southern, tropical region (Suhum District). (4) There will be a lower level of E. coli contamination in boreholes not associated with risk factors. (5) There will be a lower level of E. coli contamination in boreholes than in household storage units for water. A further goal of this study is to evaluate the effectiveness of the Portable Microbiology Laboratory and to share these methods with governmental agencies that are involved in water quality and with non-governmental organizations (NGOs) that sponsor drinking water projects. This research has several original components. Ghana government agencies that have responsibility for water-quality monitoring rarely perform any tests in rural areas, and the tests they do perform are the inexact and cumbersome thermotolerant tests. My project is the first extensive use of the Portable Microbiology Laboratory in rural Ghana, and the first attempt to involve community members in Ghana in the testing of their water sources. This study has introduced Ghana government agencies to innovative methods for testing E. coli in water in rural areas. The demonstration in this study of the usefulness 17 and practicality of the Portable Microbiology Laboratory may encourage adoption of these tests by government agencies, leading to significant improvements in public health in Ghana. 18 MATERIALS AND METHODS Study Sites and Sample Collection The two study sites for this research were the Suhum-Kraboa-Coaltar (Suhum) District, Eastern Region, Ghana and the Nanumba-North (Nanumba) District, Northern Region, Ghana. The Suhum District is located at latitude 6 N. in a moist, semideciduous forest, Celtis-Triplochiton Association, and partially includes a rain forest transition zone. The Nanumba District is located at latitude 9 N. in the Guinea Savannah Woodland. Twenty-five communities were selected in each district. All available (functioning) point sources of water in each community were sampled, typically one to four point sources per community. Point sources included boreholes (deep drilled wells with hand pumps, >50 m), shallow hand-dug wells (<30 m) with and without hand pumps, a rainwater collection system, and ponds. Additionally, two rivers used as water sources were also tested as shallow, open sources. An emphasis was placed on sampling boreholes. Flaming was not used to sterilize the pump outlet, because the desire was to know the quality of water being collected by the users. For wells with bucket retrieval, a sterile, 4 oz. stand up Whirl-Pak (Nasco, Modesto, California) was filled from the bucket typically used by the community for this purpose, or by lowering the Whirl-Pak on a rope into the well. In the Suhum District, 33 samples from unique boreholes were collected during the short rainy season (October). In the Nanumba District, samples were collected from 47 unique boreholes, twice from each of 32 point sources, including 40 samples in the dry season (March) and 39 samples in the rainy season (June). One sample from the 19 drinking water supply in the household closest to the primary point source was tested in each community in the Nanumba District during the rainy season. The household closest to the borehole was chosen to be tested in an attempt to limit the impact of introducing water from multiple sources into the storage unit, which could have residual impact on water quality. Colilert Defined Substrate Medium (10 ml test) The detection of coliform and E. coli in 10 ml and 100 ml samples was performed using the Colilert Defined Substrate Medium (IDEXX Laboratories, Westbrook, Maine). The Colilert media contains no organic sources of nitrogen and only two carbon sources as energy (ONPG and MUG), which discourages the growth of most heterotrophic bacteria. The enzyme -galactosidase for metabolizing lactose is an inducible enzyme produced by all coliform bacteria, including E. coli. This enzyme also hydrolyzes the colorless carbon source Ortho-nitro-phenol-β D-Galactopyranoside (ONPG) into the sugar galactopyranoside (G) and o-nitrophenol (ONP), which has a bright yellow color (11). The yellow color indicates the presence of coliforms. The enzyme -glucuronidase is constitutively expressed by greater than 95% of all E. coli strains, and can hydrolyze the colorless carbon source 4-methyl-umbelliferone β-D-Glucuronide (MUG), forming the fluorescent product 4-methyl-umbelliferone (MU) and a sugar (11). MU fluoresces under UV light (340 nm). E. coli is the only coliform that constitutively produces β-glucuronidase, although strains of bacteria in the genus 20 Salmonella, Aerococcus, Bacillus and Klebsiella have also been shown to have βglucuronidase activity (6). When grown in Colilert media, a pure culture of E. coli will first consume the MUG, causing the tube to fluoresce, and then consume the ONPG, causing the tube to turn yellow. Without E. coli, or when a different coliform dominates in a mixed culture, the β-D-galactopyranoside will be consumed first, causing the tube to appear yellow before it fluoresces. Glass tubes, pre-dispensed with Colilert media from the manufacturer, were marked to indicate a 10 ml volume of water. Water collected in the sterile Whirl-Pak was immediately transferred to the glass tubes using a sterile pipette. The tube was then incubated against the body of the author (approximately 37C). After 16-24 hours, the tube was checked for the presence of E. coli, as indicated by fluorescence when a longwave, battery-operated UV light was shown on it. If the 10 ml sample were negative for ONPG and/or MUG, the 10 ml was transferred to the reserved 90 ml sample in the sterile Whirl-Pak. The Whirl-Pak was placed in a black bag in the sun for 18 hours. This provided a test for the presence or absence of E. coli in 100 ml, the standard for very-low-disease-risk or treated water. The method of testing 100 ml was developed by the author in the field and has several limitations, discussed supra in a section on quality assurance, that place it outside of the guidelines provided by the manufacturer. 21 E. coli Count Petrifilm (1.0 ml test) E. coli Count Petrifilm (3M Microbiology Products, St. Paul, Minnesota) was used for detection of coliform and E. coli in 1 ml of water. In references hereafter, the Petrifilm test is referred to as the 1.0 ml test and the above described Colilert test is referred to as the 10 ml test. The E. coli Count Petrifilm includes violet-red bile nutrients that consist of lactose, a tetrazolium indicator for gram-negative bacteria, and a glucurondiase indicator (BCIG, 5-bromo-4 chloro-3 indolyl-β D Glucuronide) to identify E. coli. Non-E. coli coliform bacteria appear as a red colony surrounded by a gas bubble, which is formed from the fermentation of lactose. Among the coliform bacteria, only E. coli constitutively express β-glucuronidase and can hydrolyze BCIG to form the blue precipitate BCI. Thus, the E. coli appear as a blue colony surrounded by a gas bubble. In the Petrifilm test, after removing the film from the foil packet, the pack was resealed with masking tape to prevent moisture from entering. Immediately after collection of water from the source using a Whirl-Pak, the E. coli Count Petrifilm was inoculated with one ml of water from the Whirl-Pak, using a sterile pipette. The water was distributed over the circle of nutrients using a plastic spreader and allowed to solidify for one minute. The Petrifilm was placed between two pieces of thin, firm cardboard to prevent bending. The stacks of up to 16 Petrifilm were incubated on the body of the author (approximately 37C) for up to 24 hours. Additional supplies for the Portable Microbiology Laboratory include a sterile 4oz Standup Whirl-Pak (Nasco, Modesto, California) to collect a water sample, an 22 individually wrapped, 3.5 ml graduated, sterile pipette (Evergreen Scientific, Los Angeles, California), and a battery-operated, long wavelength UV lamp (Spectronics Corp., Westbury, New York). Visual Sanitation Survey of Boreholes At each borehole, a visual sanitation survey of the point source was conducted and recorded according to standard guidelines (16). At least one community person was surveyed for demographic information about usage of the water source and for his or her opinion of the water quality. A priority was placed on interviewing a local representative of CWSA followed by an adult individual from among those (principally women) who used the point source. A blank survey can be found in Appendix C. An additional question regarding the erosion level was added after the first round of testing, when a chance observation of erosion was linked with contamination. A score was given to each borehole based on the number of points of concern (014) identified (Appendix C). The boreholes were grouped into three levels of concern based on their score: 0-3 (lowest concern), 4-6, or 7-9 (highest concern). Statistical Analysis The results of the Colilert and Petrifilm tests were combined and interpreted to assign each source with a disease-risk level (Table 1). For various groupings of sources, a count of the point sources with each disease-risk level was compiled. A Chi-square test 23 for goodness of fit was used to determine if, for a particular source type, such as boreholes, the range of contamination varied from a random (even) distribution among the disease-risk categories of low, moderate, high, and very high. A Chi-square test of association was used to compare pairs of groupings such as boreholes/hand-dug wells, rainy/dry seasons, or north/south regions. The test was to determine if the pairs of groupings were meaningfully associated with any variation in the range of contamination. Microsoft Excel was used for all statistical analysis. The statistical significance level used was 0.05. Quality Assurance Manufacturer guidelines for the Colilert test state that after 28 hours of incubation the compounds for suppression of non-coliform bacteria are less effective. Negative results can be confirmed after 28 hours; however, positive results should be considered tentative and the sample should be retested. Because of the difficulty and time involved in the collection of each sample, tubes that appeared positive after 28 hours, and thus constituted tentative results, were not retested. Because incubation temperatures may not have been consistent, growth rate may have been lower than under manufacturer guidelines, therefore, tentative results were noted during the initial data collected, but reported as negative in the results of this study. Additionally, a few samples tested positive for MUG but negative for ONPG within the 28-hour incubation time. E. coli constitutively produces β-glucuronidase, but can be induced to produce β-galactosidase when all of the MUG sugar has been 24 consumed. Therefore, a sample that is predominantly E. coli and has few or no other coliforms will fluoresce before it appears yellow. However, the tube will eventually turn yellow and, according to manufacturer guidelines, this should occur within 28 hours. Samples that fluoresced within 28 hours but showed yellow after more than 28 hours were considered tentative but negative for E. coli; samples that fluoresced within 28 hours but never turned yellow were considered an anomaly and not considered positive for E. coli. Significant limitations occur with the 100 ml adaptation of the Colilert test. No follow-up testing to confirm these results against standard and approved methods was done. Because these 100 ml samples were incubated without temperature control and contained diluted nutrients, potential false negatives could occur. The average ambient temperature in Ghana is 26°C. Seasonal ranges for the regions where testing took place are as follows: the range near Accra (closest major city to Suhum District) in October is 22-30°C, the range near Tamale (closest major city to Nanumba District) in March is 2437°C, and in June is 22-31°C. Potential false positives also could occur with this method (and are perhaps more likely) because inhibitors of non-coliform growth may be insufficient. In the Nanumba District during the second testing phase (June rainy season), the 100 ml sample was inoculated simultaneously with the 10 ml sample using the amount of reagent from a 10 ml tube. While this version is superior because it avoids the breakdown of inhibitors that occurs after 28 hours, according to the manufacturer, it is still limited by the factors noted above. 25 RESULTS In order to evaluate the relative disease risk of boreholes as compared to hand-dug wells in Ghana, I tested samples from boreholes, hand-dug wells and other sources in two regions of the country during two seasons. The results set forth extend to new data points beyond what Ghana’s rural water agency (CWSA) has obtained in the past in its recommended routine of testing two boreholes per year in each district using the thermotolerant test. The Portable Microbiology Laboratory used in this study enabled the collection of this new data and allowed for community participation (Figure 1). 26 Figure 1. Using the Portable Microbiology Laboratory in Ghana. District Water and Sanitation Team member Gabriel M. Nartey uses a sterile pipette to transfer possibly contaminated drinking water from a borehole in Aponopono, Suhum District to the 10 ml Colilert tube in his left hand. The red 1 ml Petrifilm and Whirl-Pak are resting on the head of the borehole pump. 27 Contamination Levels in Hand-dug Wells and Other Shallow Sources To evaluate the disease-risk level of hand-dug wells and other open sources, I tested their bacterial contaminations levels. I obtained 33 samples from hand-dug wells in the Suhum and Nanumba districts (Table 2). In the Suhum District, these consisted of 22 samples from open hand-dug wells and eight samples from hand-dug wells with pumps. In the Nanumba District, the samples consisted of three from open hand-dug wells and one from a hand-dug well with a pump. E. coli and coliforms were consistently detected in drinking water from hand-dug wells using the Petrifilm and Colilert tests. Specifically, E. coli and coliforms were detected in all 22 open hand-dug wells in the Suhum District in both 10 ml and 1.0 ml tests (Table 2, Figure 2). E. coli were detected on the Petrifilm tests at rates of contamination between 1 and 60 cfu/ml of water, which is equivalent to a World Health Organization disease-risk level of high or very high (Table 1). As would be expected, more non-E. coli coliform than E. coli were counted in 31 of 34 sources, which confirms the benefit of using the testing method that evaluates specifically for the presence of E. coli. Two of the hand-dug wells (Siriki and Otwebediadua #1) had more than twice as many E. coli as coliforms (Table 2). In all, eight hand-dug wells with a pump were tested in Suhum; coliforms were detected in both 10 ml and 1.0 ml samples (Table 2, Figure 2). E. coli were detected in a 1.0 ml sample in four of the eight hand-dug wells with pumps (50%). In the 10 ml samples, E. coli were detected in six of the hand-dug wells with pumps (75%). One handdug well with a pump where E. coli were not detected had been recently chlorinated (Gabriel M. Nartey, Suhum District Water and Sanitation Team, personal 28 communication). High levels of environmental coliforms were detected in all hand-dug wells both with and without a pump. In addition to the samples of drinking water from hand-dug wells, the study obtained six samples from other open sources (Table 3, Figure 2). In Suhum, the study obtained one sample from a rainwater collection system (positive for E. coli in a 10 ml sample, moderate disease risk). The study obtained one sample from an open stream (positive for E. coli in a 1.0 ml sample, high disease risk). In the Nanumba District, E. coli colonies were detected in 1.0 ml samples in three of the four open sources (two lakes and a river), which were therefore classified as high disease risk. While low disease-risk drinking water was detected on occasion from shallow, hand-dug wells (based on the WHO disease-risk levels), a majority of the sampled handdug wells and other open water sources had high and very high disease-risk levels. A Chi-square goodness-of-fit test (2) statistically confirmed the observation. The Chisquare test confirmed an uneven (different from random) distribution of the total number of hand-dug wells (with and without pumps) in the Suhum District at each disease-risk level: two wells were low disease risk; two were moderate disease risk; 14 were high disease risk, and 11 were very high disease risk (Figure 2, 2=14.4, p<<0.001). The Chi-square test cannot be used meaningfully on the data from the hand-dug wells with pumps alone, because while it meets a requirement for the average expected frequency of at least 2.0, Koehler and Larntz suggest that the total number should be greater or equal to 10, and the study tested only eight hand-dug wells with pumps (21). 29 The WHO disease-risk level observations from the few available samples of open water sources other than hand-dug wells (two at moderate disease risk, four at high disease risk) suggest that the disease risk of these other open sources being contaminated may be similar to the pattern found for hand-dug wells. It may be that the majority of open sources are high disease risk. However, beyond this general suggestion, the study collected insufficient data to make a statistical analysis of whether the six open sources tested were more or less risky than other point sources. A Chi-square test combining the observations from all these surface and shallow point sources (hand-dug wells with and without pumps, rainwater collection, dammed lakes, and rivers) produced results consistent with the previous suggestion that all of these sources trend towards higher levels of contamination. Statistically, they are also unevenly distributed (Figure 2, 2= 19.8, p<0.001). 30 Table 2. Test results for drinking water from shallow, hand-dug wells. Community Source1 Petrifilm Colilert Suhum District Nsuta Wawase Okanta Kwabena Kumi Adarkwa Siriki Otwebediadua #2 Ntunkum Jato Kudzi Akorabo Manfe Nkwata Amenhia Amede Wekpeti Ali Kaponya Nkwata Anoma Otwebediadua #1 Sawa Amenhia Nanumba District Bimbla D Section Bimbla Imam Chiriloyili 1 Coliform (non-E. coli) E. Coli ONPG MUG HDW2 HDW HDW HDW 1 HDW 2 HDW 3 HDW HDW 1 HDW 2 HDW HDW HDW 1 HDW 2 HDW 3 HDW 4 HDW 5 HDW 6 HDW HDW 1 HDW 2 HDW HDW HDWp3 1 HDWp 2 HDWp HDWp HDWp 1 HDWp 2 HDWp HDWp TNTC4 20 TNTC 7 30 9 10 10 51 9 51 43 + 17 +5 + + 7 + 22 32 + + 2 41 5 8 2 12 TNTC 11 5 61 3 1 1 20 3 58 8 12 28 2 5 9 15 2 1 7 1 14 TNTC 0 0 3 0 17 0 1 88 + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + HDW 1 HDW 2 HDWp HDW 1 17 0 TNTC 0 0 0 8 WHO Disease-risk Level Very High High Very High High High High Very High High Very High High Very High Very High High High High Very High High High High High Very High Very High Moderate Low*6 High Moderate Very High Low High Very High Moderate/Low Moderate/Low Moderate/Low High Source (e.g., 1, 2, or 3) references the first, second, or third site tested in the community identified. HDW = open, shallow, hand-dug well with bucket retrieval. 3HDWp = well capped with a pump. 4 TNTC = too numerous to count, assumed >100 cfu. 5 + (on Petrifilm) = coliform colonies were detected but not counted. 6 Low* = sample was also MUG negative in 100 ml, indicating very low disease risk. 2 31 100% Percentage of wells tested 90% 80% 2 9 11 70% High Disease Risk 2 60% Very High Disease Risk 50% 40% 30% 2 14 2 2 2 12 20% 10% 0% Hand-dug wells without pumps Moderate Disease Risk Low Disease Risk Hand-dug wells with pumps All hand-dug wells with and without pumps Hand-dug wells in the Suhum District Figure 2. Surface and shallow water sources at each WHO disease-risk level in the Suhum District. The number in each column section represents the number of sources at each WHO disease-risk level. 32 Table 3. Test results for drinking water from open water sources. Community Source Petrifilm Colilert Coliform (non E. coli) E. coli ONPG MUG + + WHO Disease-risk Level Suhum District Kwabena Kumi Rain 2 0 Moderate Kwabena Kumi River 12 3 High Bimbla Lake Lake 11 3 High Champa Dam Lake 20 0 Moderate/Low Gbeini Nakpa Dam Lake 35 4 High Yapala River River 30 7 High Nanumba District 33 Contamination Levels in Boreholes To evaluate the disease-risk level of boreholes, I tested their bacterial contamination levels. For drinking water from deep, drilled boreholes, both coliforms and E. coli were detected using the Colilert and Petrifilm methodologies with 10 ml and 1.0 ml samples. The study obtained 112 samples from 80 boreholes. Of these, 33 of the boreholes were from the Suhum District (Table 4) and 47 of the boreholes were from the Nanumba District (Table 5). E. coli were not detected in either the 1.0 ml or 10 ml samples in any of the 33 boreholes tested in the Suhum District (Table 4). E. coli were detected in a 1.0 ml sample in one borehole at Karalga in the Nanumba District during the rainy season (Table 5). In total, E. coli were detected in 10 ml samples in 10 boreholes from Nanumba (21% of the samples from the district). The 10 ml sample testing detected E. coli in five boreholes during the dry season (13% of Nanumba boreholes tested that season) and seven boreholes during the rainy season (18% of Nanumba boreholes tested that season). Of the 10 Nanumba boreholes that tested positive for E. coli, two boreholes tested positive for E. coli during both the rainy and dry seasons (Table 5). Turning from detection of E. coli in the boreholes to detection of coliforms, the Suhum District testing detected coliforms in 1.0 ml samples in four boreholes (12% of the boreholes tested in the district) and in 10 ml samples in nine boreholes (27% of the boreholes tested in the district) (Table 4). Coliforms were detected in 1.0 ml samples in 15 Nanumba boreholes (32% of the boreholes tested in the district). Of these 15 Nanumba boreholes, coliforms were detected 34 in seven (17.5%) during the dry season and nine (23%) during the rainy season. Coliforms were detected in a 1.0 ml sample in one borehole during both seasons (Table 5). Coliforms were detected in 10 ml samples in 28 boreholes (60%) from the Nanumba District. Of 28 boreholes, coliforms were detected in 12 (30%) during the dry season and in 20 (51%) during the rainy season. Coliforms were detected in 10 ml samples from four boreholes during both seasons (Table 5). The trend among boreholes was to be low disease risk. This was statistically confirmed with a Chi-squared goodness-of-fit test that showed that the number of boreholes at each disease-risk level was unevenly distributed. In the Suhum district, 27 boreholes (82%) were very low disease risk, and six boreholes (18%) were low disease risk (Figure 3, 2= 13.4, p<0.001). In the Nanumba District, the distribution of boreholes among the various disease-risk levels followed a similar pattern during both the dry and rainy seasons. In the Nanumba dry season, 31 boreholes (78%) were very low disease risk, three (7.5%) were low disease risk, and six (15%) were moderate disease risk (Figure 3, 2=44.5, p<<0.001). In the Nanumba rainy season, 27 boreholes (69%) were very low disease risk, five (13%) were low disease risk, six (15%) were moderate disease risk, and one (3%) was high disease risk (Figure 3, 2=50.9, p<<0.001). The distribution of 72 independent borehole samples from both districts during the rainy season at each disease-risk level was uneven, 63 were low (or very low) disease risk, six were moderate disease risk, and one was high disease risk (Figure 3, 2=238, p<<0.001). 35 Table 4. Test results from boreholes in the Suhum District during the short rainy season (October). Community Source1 24 hr Petrifilm Colilert WHO Coliform E. Coli ONPG MUG Disease (non-E. coli) Risk Level2 Omenako Obretema Krobomu Aponopon Nsuta Wawase Suhum Tech Ateibu Okanta Kwabene Kumi Adarkwa Ayisikrom Kweadjo Owuo Akote Ntunkum Abisim Dawa Abisim Adjatey Kwahia Jato Jato Jato Kudzi Akorabo 1 1 1 2 1 1 2 1 2 1 2 1 2 3 1 1 1 1 1 1 1 1 2 1 1 2 3 1 2 1 2 1 2 3 0 0 0 0 0 0 0 0 0 0 0 0 0 1 0 0 0 0 0 0 1 2 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 + + + + + faint + faint + + + - - Low* Low* Low* Low* Low* Low* Low* Low* Low* Low Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low Low* Low* Low Low* Low* Low* Low* Low Low Low Low* Source (e.g., 1, 2, or 3) references the first, second, or third site tested in the community identified. 2 Low* = sample was also MUG negative in 100 ml, indicating very low disease risk. 36 Table 5. Test results for boreholes in the Nanumba District during both the dry (March) and rainy (June) seasons. Community Source1 Season 24 hr Petrifilm Colilert WHO Coliform E. ONPG MUG Disease(non-E. coli risk coli) Level2 Bimbla D Section Bimbla Jekpafuri Bimbla Badiligli Bimbla Imam 1 2 1 1 1 Dakpam 2 1 2 Nabagnando Chamba – Small London Gungunpaya 3 1 1 1 2 Jangbojado 1 Afayili Jayindo #2 Afayili Jekodo 1 1 Chamba - police 2 3 1 Manchoni 1 2 Kpabi 3 1 2 1 Dry Dry Rainy Rainy Dry Rainy Dry Dry Rainy Dry Rainy Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry 1 6 0 0 1 0 0 0 0 0 0 0 7 0 0 0 0 0 0 0 5 0 1 0 Dry Rainy Rainy Rainy Dry Rainy Dry Rainy Dry Rainy Dry Dry Rainy Dry 0 10 2 1 0 3 0 0 0 - 0 0 0 0 0 - + + + + + + + + + + >24 + + + + faint + + + + faint + faint + + - Low* Low* Moderate Low* Low* Low* Low* Low* Low* Low Low Low* Low Low* Low* Low* Moderate Low* Moderate Moderate Low* Low Low* + + + faint + - Low Low* Low* Low Moderate Moderate Low* Moderate Low* Low* Moderate Low* Low* Low* Source (e.g., 1, 2, or 3) references the first, second, or third site tested in the community identified. 2 Low* = sample was also MUG negative in 100 ml, indicating very low disease risk. 37 Table 5 continued. Community Source1 Season Pusuga 1 2 3 Demonayili 1 2 Chiriloyili 3 1 Gilisya 1 2 Kpaligaboni 1 Dipa 1 2 Gbeini Nakpa 1 Yapala 2 1 Chakosi / Jakpafoli 1 Karalga Juo 1 Jua 1 Juasheya 1 Guidua #2 2 1 Guidua #1 1 1 Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry Rainy Rainy Dry Rainy Dry Dry Rainy Dry Rainy Dry Rainy Dry Rainy Dry Dry Rainy Dry Rainy 24 hr Petrifilm Coliform (non-E. coli) 0 0 2 1 1 0 2 + 4 3 0 14 - E. coli 0 1 2 0 - Colilert ONPG + + + + + + + + + + + + + + + + + MUG + + + + + - WHO Diseaserisk Level2 Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Moderate Low* Low* Low Low* Moderate Low* Low* Low* Low* Low* Low* Low Low* Low* Low* Low* High Low* Low* Low* Low* Low* Low* Moderate Low* Moderate Low* Low* Source (e.g., 1, 2, or 3) references the first, second, or third site tested in the community identified. 2 Low* = sample was also MUG negative in 100 ml, indicating very low disease risk. 38 100% Percentage of boreholes tested 90% 80% 33 1 6 34 6 32 High Disease Risk 70% 60% Moderate Disease Risk 50% 40% 30% Low Disease Risk 20% 10% 0% Suhum District, Nanumba District, Nanumba District, rainy season dry season rainy season Boreholes by region and season Figure 3. Comparison of boreholes by region and season at each WHO disease-risk level. The number in each column section represents the number of sources at each WHO disease-risk level. 39 Visual Sanitation Survey of Boreholes In order to evaluate the correlation between established visual indicators of contamination and the levels of E. coli in boreholes, I also performed a visual sanitation survey. The visual sanitation survey identified possible sources of fecal contamination or pathways for fecal contamination to enter the groundwater or point source. No borehole scored more than nine out of 14 possible points. No correlation was found among the distribution of boreholes at each WHO disease-risk level in these three levels of concern (Figure 4). Novel Testing Method for 100 ml Samples During the sampling and analysis of the well water, it seemed possible to expand the use of the Portable Microbiology Laboratory by adding an additional step to enhance the sensitivity of the testing methods. To evaluate the samples further, therefore, I tested for the presence or absence of E. coli in 100 ml of water as detailed in the Materials and Methods section. With the addition of the 100 ml Colilert sample data, further comparisons can cautiously be made, noting the limitations previously stated. Among 99 borehole samples that tested MUG negative in 10 ml samples, 85 (86%) were also MUG negative in 100 ml samples (Figure 5). 40 20 18 18 1 16 Number of boreholes High Disease Risk 3 14 13 12 10 2 8 9 6 7 6 4 2 3 Moderate Disesae Risk Low Disease Risk 0 0-3 4-6 7-9 0-3 4-6 7-9 (low risk) (moderate) (high risk) (low risk) (moderate) (high risk) Suhum Nanumba Visual Sanitation Survey Score for boreholes by district Figure 4. Comparison of boreholes at each WHO disease-risk level for various score ranges on the sanitation survey. The number in each column section represents the number of sources at each WHO disease-risk level. Percentage of boreholes tested 41 100% 90% 80% 70% 6 3 31 27 5 27 Low Disease Risk (100 ml MUG pos.) 60% 50% 40% Very Low Disease Risk (100 ml MUG neg.) 30% 20% 10% 0% Rainy Season Dry Rainy Suhum District Nanumba District Boreholes by distric and season that are MUG negative in 10 ml Figure 5. Comparison of Colilert test results from 10 ml and 100 ml borehole samples. The number in each column section represents the number of sources at each WHO disease-risk level. 42 Independence of Well Sources Within a Community For villages with multiple wells in the same village, I examined, with the inclusion of the 100 ml data, how many villages had wells that were consistent and how many villages had wells that differed in disease-risk level. Overall, more villages showed variation (19 villages) between their sources than had the same quality in all wells (16 villages) (Table 6). Hand-dug wells and boreholes in the Nanumba District dry season both had sources that ranged across four different disease-risk levels, and both of these situations had more villages that showed variation. Boreholes in Suhum only spanned two disease-risk levels and Nanumba (rainy season) spanned three disease-risk levels. Also, these locations did not have as much variation within villages. Comparison of Boreholes and Hand-dug Wells I used a test of association (2) to compare various sources to each other. The disease-risk category distribution for boreholes and hand-dug wells in the Suhum District was significantly different, (Figure 6, 2=42.7, df=2, p<<0.001), with hand-dug wells more often in the higher disease-risk categories than boreholes. Inclusion of the Nanumba District data does not change these results. However, this study presents only the Suhum District data in this comparison in order to avoid variability that might theoretically be caused by differences in geography or season (though no such variability was in fact observed). 43 Table 6. Comparison of disease-risk level of water sources within and between communities. Source Same diseaseDifferent risk level disease-risk level Hand-dug wells (7 communities, Suhum District, rainy season) Boreholes (8 communities, Suhum District, rainy season) Boreholes (11 communities, Nanumba District, dry season) Boreholes (9 communities, Nanumba District, rainy season) Total 2 5 4 4 7 4 3 6 16 19 44 100% Percengate of sources tested 90% 80% 33 11 70% Very High Disease Risk High Disease Risk 60% 50% 40% 14 30% Moderate Disesae Risk 20% 10% 2 0% 2 Low Disease Risk Boreholes Hand-dug wells Water source type in the Suhum District Figure 6. Comparison of boreholes and hand-dug wells in the Suhum District at each WHO disease-risk level. The number in each column section represents the number of sources at each WHO disease-risk level. 45 Comparison of Disease Risk Across Geographical Variation Disease-risk category distribution was significantly different for boreholes in the two distinct geographical zones represented by the Suhum and Nanumba districts, with boreholes in the Suhum District more consistently being low risk than boreholes in the Nanumba District (Figure 3, 2=6.6, df=2, p=0.038). Only data from the rainy season in Nanumba were used in order to hold constant for seasonal variation (though no such variation could be confirmed by this study). Comparison of Disease Risk Across Seasonal Difference In order to evaluate the variation in disease-risk level over a three-month period, dry and wet season paired samples were taken for 32 boreholes in the Nanumba District (Figure 7). While there were more high and moderate disease-risk boreholes during the rainy season than in the dry season, there was no significant difference between the distribution of disease risk for the two seasons (Figure 7, 2=1.19, df=2, p=0.55). A more detailed evaluation shows that 16% (5/32) of boreholes changed over this three-month period: three boreholes increased from low to moderate disease risk and one borehole increased from low to high disease risk (two orders of magnitude) from the dry to the rainy season, while one borehole decreased from moderate to low disease risk from the dry to the rainy season (Table 7). Two boreholes were moderate disease risk during both seasons. Inclusion of the 100 ml data adds an additional five boreholes that changed between low and very low risk from season to season, increasing the number of boreholes that changed over the three months to 31% (10/32). Percentage of boreholes tested 46 100% 1 90% 5 80% 70% 26 4 28 60% High Disease Risk Moderate Disease Risk 50% 40% 30% Low Disease Risk 20% 10% 0% Rainy season Dry season Boreholes tested twice in the Nanumba District Figure 7. Comparison of boreholes at each WHO disease-risk level in the Nanumba District during two seasons, rainy and dry. The number in each column section represents the number of sources at each WHO disease-risk level. 47 Table 7. Comparison of WHO disease-risk levels in paired water test results for boreholes in the Nanumba District during two seasons, dry and rainy. Community Dry1 Rainy Order of magnitude change in number of E. coli in sample2 Bimbla Imam Section Dakpam Nabagnando Chamba – Small London Gungunpaya Jangbojado Afayili Jekodo Chamba - police Manchoni Kpabi Pusuga Demonayili Chiriloyili Gilisya Kpaligaboni Dipa Gbeini Nakpa Yapala Karalga Juo Jua Juasheya Guidua #2 Guidua #1 1 Low* Low* Low Low Low* Mod Mod Low* Low Mod Low* Low* Low* Low* Low* Low* Low* Low* Low* Low Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low* Low Low* Low* Low* Mod Low Low* Mod Mod Low* Low* Low* Low* Low* Low* Low* Low* Low* Low Mod Low* Low* Low* Low* High Low* Low* Low* Mod Low* Same Same Same Same3 Same +1 Same Same3 Same3 Same -1 Same Same Same Same Same Same Same Same Same3 Same3 -1 Same Same Same Same -2 Same Same Same -1 Same Low* = sample was also MUG negative in 100 ml, indicating very low disease risk. The order of magnitude change reported in the column represents any difference in disease-risk level from the dry season to the rainy season. Every disease-risk level change represents a 10-fold change in the quantity of bacteria cfu in the sample. 3 Boreholes that are designated “Same3” met the tolerable disease-risk level of no E. coli in 10 ml during both seasons, however, there was a disease risk change from very low to low or vice-versa, as explained in footnote 1. 2 48 Contamination Levels in Household Water Storage Units In order to evaluate the relative disease risk of water directly from the borehole source as compared to water from the borehole after it has been placed in storage in nearby homes, I tested the bacterial contamination levels in household storage units. Both coliform and E. coli were detected in household water storage units using the Petrifilm and Colilert methodologies. Twenty-three samples were taken from household water storage units in the Nanumba District during the rainy season (Table 8). E. coli were detected at 1.0 ml in 30% (7/23) of households, and at 10 ml in 74% (17/23) of households. Coliforms were detected at 1.0 ml in 65% (15/23) of households and in all 23 households at 10 ml. The total number of household supplies at each disease-risk level was unevenly distributed (Figure 8, 2=9.5, p=0.02). Twenty-one paired samples of boreholes and the closest available household water storage unit were taken. Disease-risk category distribution was significantly different between the source boreholes and the household supplies for these paired samples (Figure 8, 2=15.1, df=2, p=0.0005), with households having higher disease-risk levels than boreholes. A closer look, with the inclusion of 100 ml Colilert samples, shows that 14% (3/21) of households maintained the same water quality in household storage as at the source (one very low disease risk, two moderate disease risk), 29% (6/21) of households were ten times more contaminated than the source (four changed from very low to low, one changed from low to moderate, one changed from moderate to very high), 48% (10/21) of households were two orders of magnitude more contaminated than the source 49 (seven changed from very low to moderate, two from low to high, one from moderate to very high), and 10% (2/21) of households were three orders of magnitude more contaminated than the source (very low to high disease risk) (Table 9). No household improved the water quality from the source. 50 Table 8. Water test results from household storage units in the Nanumba District, rainy season. Community 24 hr Petrifilm Colilert WHO Disease-risk Coliform E. coli ONPG MUG Level Dakpam Nabagnando Chamba – Small London Gungunpaya Jangbojado Afayili Jayindo #2 Afayili Jekodo Manchoni Kpabi Pusuga Demonayili Chiriloyili Gilisya Kpaligaboni Dipa Gbeini Nakpa Yapala Juo Jua Juasheya Guidua #2 Guidua #2 1 (non E. coli) TNTC1 26 2 1 0 TNTC +2 32 + 6 + 14 21 TNTC 43 + 2 - 0 20 2 5 0 4 0 0 0 0 1 0 0 2 0 0 0 0 0 0 + + + + + - Moderate High Low + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + Moderate Very High High High Low High Moderate Moderate Low Low High Moderate Moderate High Low*3 Moderate Moderate Moderate Moderate Moderate TNTC = too numerous to count, assumed >100 cfu. + (on Petrifilm) = coliform colonies were detected but not counted. 3 Low* = sample was also MUG negative in 100 ml, indicating very low disease risk. 2 Percentage of samples tested 51 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% 4 6 High Disease Risk 17 10 5 Moderate Disease Risk Low Disease Risk Borehole Household drinking supply Source and storage of water in the Nanumba District, rainy season Figure 8. Comparison of closest boreholes and household drinking supplies in Nanumba District (rainy season) at each WHO disease-risk level. The number in each column section represents the number of sources at each WHO disease-risk level. 52 Table 9. Order of magnitude change comparison of contamination levels in households to the contamination level at the nearest borehole. Community Level of Level of Order of contamination in contamination in magnitude borehole household change in storage unit number of E. coli in sample1 Dakpam Nabagnando Chamba – Small London Gungunpaya Gungunpaya Jangbojado Afayili Jayindo #2 Afayili Jekodo Manchoni Kpabi Pusuga Demonayili Chiriloyili Gilisya Kpaligaboni Dipa Gbeini Nakpa Yapala Juo Jua Juasheya Guidua #2 1 Very Low Very Low Very Low Moderate High Low -2 -3 -1 Very Low Moderate Low Very Low Very Low Moderate Low Very Low Very Low Very Low Low Moderate Very Low Very Low Very Low Very Low Very Low Very Low Moderate Moderate Very High High High Low High Moderate Moderate Low Low High Moderate Moderate High Very Low Moderate Moderate Moderate Moderate -2 -2 -2 -3 -1 -1 -1 -2 -1 -1 -2 Same -2 -3 Same -2 -2 -2 Same The order of magnitude change reported in the column represents any difference in disease-risk level from the dry season to the rainy season. Every disease-risk level change represents a 10fold change in the quantity of bacteria cfu in the sample. 53 DISCUSSION The results of this study substantiated, using a new technique, the presence of the fecal indicator bacterium E. coli in various point sources of drinking water in rural Ghana. Further, the results verified the strategy of CWSA of providing low-disease-risk drinking water (defined in this study and by WHO as free from E. coli contamination in 10 ml) through the construction of boreholes. Most of the samples taken from boreholes in the two regions during various seasons (88%, 99/112) represented low disease risk, but only 6% (2/34) of samples from hand-dug wells met this standard. This study concluded that, in these regions of study in Ghana where almost no microbiological testing of water had been performed up to this point, the prevalence and levels of E. coli were highest in shallow, unprotected water sources and lowest in boreholes, which provide a deep source of water. Only one borehole was identified as high disease risk (allowing for corrective action). Among the 11 boreholes in the Nanumba District that were not low disease risk, there was not a significantly greater number in the rainy or dry season. Eighty six percent (20/23) of household storage units had higher levels of E. coli than the corresponding borehole, suggesting that in addition to encouraging use of boreholes over hand-dug wells, attention needs to be given to how households handle water. Taking the results from this study as a snapshot of a water system composed of all point sources examined, the data in this study could be used to validate the performance of each district in meeting policy of the CWSA. Using “no E. coli in 10 ml of water” as the tolerable risk level, all boreholes in the Suhum District met this standard, and the 54 district performance can be rated as excellent. For the Nanumba District, 76% (35/46) of the boreholes met this standard, and the district performance can be rated as moderate. I further demonstrated that use of the Colilert and Petrifilm tests enabled low-cost, convenient, and efficient data collection and assessment of rural water sources. This combination of drinking water tests in the Portable Microbiology Laboratory was effective in establishing the prevalence and levels of contamination, in line with WHO disease-risk classifications of low, moderate, high, or very high. The demonstrated effectiveness of the testing techniques gives the CWSA, for the first time, a viable opportunity to collect a substantial amount of data in order to set water policy guidelines based on actual contamination rates. The results from this study demonstrated changes in water quality over a short period of time in boreholes (Table 7). Additionally, because a range of contamination levels (including low, moderate, and high disease risk) were found in boreholes, it appears that current CWSA policy for random sampling of two boreholes per district per year is insufficient to monitor potential health risks or to set effective policy and to verify its implementation. The Portable Microbiology Laboratory tests can be incorporated into the ongoing work with communities of CWSA officers and provide timely (within 24 hours), comprehensible feedback to help the CWSA meet the minimum WHO monitoring guidelines (39). In addition, with minimal training, the tests could also be performed and interpreted by community members for self-monitoring, which is not possible with current testing methods in Ghana (Figure 1). 55 WHO advocates a risk-assessment approach for water quality analysis. Risk analysis combines the results of E. coli counts with a sanitary inspection (38). The sanitary inspection consists of a visual analysis of factors affecting water quality and needs no equipment. What has been missing in risk analysis surveys has been testing for E. coli. The results of this study demonstrate that the Portable Microbiology Laboratory tests can fill this missing link and enable CWSA to improve its detection and management of health risks associated with drinking water. Particularly in high disease-risk areas, relatively modest, incremental improvements in water quality can result in substantial health gains (34). The Portable Microbiology Laboratory is particularly suited to identify these high and very high disease-risk sources and can be a helpful addition for establishing a baseline on which to set water policy, for verification of water policy targets for point sources, and as a component of community-based monitoring. Use of the Portable Microbiology Laboratory can help CWSA in each of these three ways in improving its early detection and management of health risks associated with contaminated drinking water. Point Sources Hand-dug Wells Without Pumps. The presence of E. coli in Portable Microbiology Laboratory tests in all 22 open hand-dug wells in the Suhum District indicates that drawing water from hand-dug wells poses a high (59%, 13/22) or very high (41%, 9/22) risk of disease. Tests of open hand-dug wells in the Nanumba District during the dry season showed that two of the three open hand-dug wells were negative for E. 56 coli at 1.0 ml. However, since the 10 ml Colilert test was not performed, it was not determined if these sources were of moderate (Colilert MUG positive) or low disease risk (Colilert MUG negative). Poor hygiene practices and other conditions observed in a sanitary survey of open hand-dug wells in the Suhum District may explain some of the high levels of E. coli in samples from these sources. Despite the active involvement of staff from the Suhum District in supporting the WatSan committees, I observed no consistent hygiene practices by the majority of district communities to maintain their well quality. Almost all studied communities, for example, used multiple buckets to fetch water from the wells, and these buckets were on occasion observed dropping on the ground and in the dirt, such that the well was repeatedly re-inoculated with environmental bacteria. The open wells were commonly exposed to sunlight and algae growth was typically visible. In several locations, community members complained of children’s negligent behavior, such as throwing sticks into the well. One well that I observed had a dead frog floating in it. Another source of contamination that could account for these high levels of E. coli is from fecal sources transported through the ground water. Open defecation was common in the villages sampled (personal observation). A number of the surveyed community representatives complained of an increase in turbidity following even the slightest rain, suggesting poor design or damage to the well casing that allowed ground water fecal sources to contaminate the wells. These observed conditions and comments were consistent with the pattern of classification in the study results for open hand-dug wells in the Suhum District as high or very high disease-risk water sources. 57 In contrast to the Suhum results, two of the three open hand-dug wells tested in the Nanumba district during the dry season had no E. coli colonies on the Petrifilm tests. All of these open hand-dug wells in the Nanumba District were a significant distance (more than 500 m) from any residence, which may have limited their exposure to fecal contamination. These results show that there are instances where open hand-dug wells can be classified as less than high disease risk and suggest a follow-up test that includes the Colilert as well as the Petrifilm test. Hand-dug Wells With Pumps. While four of the eight water samples from handdug wells with pumps indicated high or very high disease risk, the other four were classified at much lower disease-risk levels. Investigation revealed a possible explanation for the lower disease risk in some: recent chlorination. A non-governmental organization (NGO) in the Suhum District was involved in well rehabilitation, including the capping of hand-dug wells with pumps and performing shock chlorination to disinfect the water (Gabriel M. Nartey, personal communication). Several of the hand-dug wells with pumps had placards indicating this NGO was involved with their maintenance, and several local WatSan representatives noted that their wells had been chlorinated within the last two months. This study, though, was not extensive enough to demonstrate a pattern. Further study would be needed to establish whether un-chlorinated, hand-dug wells with pumps are generally high disease risk. In any event, the results show that a well maintained hand-dug well with a pump does have the potential of achieving low-disease-risk water quality. 58 Current CWSA policy is to work towards replacing all hand-dug wells with boreholes and not make further attempts at improving hand-dug wells (Charlotte Engmann, National CWSA engineer, personal communication). As a practical matter, because of the cost and time involved, the replacement of substantially all the hand-dug wells in Ghana with boreholes will likely take considerable time. In the interim, monitoring the low-disease-risk hand-dug wells with a pump using the Colilert test can provide evidence that the water source stays at a low-disease-risk level. If future Colilert tests are MUG positive, additional Petrifilm tests could be performed to evaluate the level of disease risk and re-assess mitigation options. Mitigation options to be considered, other than construction of a new borehole, could include measures such as the addition of pumps or periodic shock chlorination to improve quality. Open Sources of Drinking Water. Six open sources including small lakes (three), rivers (two) and a rainwater catchment (one) were also surveyed in this study. E. coli were present in 1.0 ml testing (high disease risk) for five of the six sources. These results are consistent with previously observed high levels of thermotolerant coliform in surface supplies of water in areas with poor sanitation in Ghana (2, 29). One lake source was classified as either moderate or low disease risk, because only the Petrifilm test was performed, and coliforms but no E. coli were present on the Petrifilm. Boreholes. The presence of E. coli at disease-risk classifications of moderate (12) or high (1) in 13 of the 112 borehole samples contradicts the general confidence expressed by staff of the CWSA that “boreholes are free from fecal contamination.” This optimistic sentiment was also reflected at the community level, where surveyed members 59 consistently thought that borehole water was high quality, although sometimes they complained of a stale or mineral taste. Testing these sources using the Portable Microbiology Laboratory is a means to identify occasional high disease risk and also to assure the community that even if the taste is different from surface water, there is a low risk of disease from fecal bacteria. The great majority of the boreholes tested (86%, 68/79) were consistently low disease risk, though the testing revealed a range of disease risk among those tested including low, moderate, and high. Overall, the success of the Portable Microbiology Laboratory in revealing a range of risk validates the effectiveness of the Portable Microbiology Laboratory in discriminating among the categories that WHO identifies as relevant. As a stand-alone assessment, the visual sanitation survey was insufficient as an indicator of fecal contaminator, as some boreholes with the highest number of indicators were still low risk when tested for the presence of E. coli. All of the boreholes with a low number of indicators on the visual sanitation were also low risk based on the absence of E. coli in 10 ml of water. Some risk factors were identified through the visual sanitation survey for all boreholes that were moderate or high risk based on E. coli levels, suggesting that the survey may be helpful with mitigation decisions even if it is insufficient as a stand-alone indicator. Boreholes (100 ml Adaptation). In developed countries where water is chlorinated, drinking water standards are for no E. coli in 100 ml, signifying a very low disease-risk level (42). As a side project with boreholes in the Suhum District, and again 60 in the Nanumba District during the dry season, I used a novel approach to look at 100 ml samples. After 24 hours of incubation, I poured the MUG negative 10 ml contents into the remaining 90 ml of the Whirl-Pak used to collect the water, which had been kept at ambient temperature. I discovered that there was enough ONPG and MUG reagent in the 10 ml sample so that ONPG and MUG tests could be detected in the Whirl-Pak with 100 ml. Following this observation, samples from the Nanumba District in the rainy season were inoculated into Colilert simultaneously at 10 ml and 100 ml using the amount of reagent present in a 10 ml tube in both cases. A cautious look at these additional data from the Nanumba rainy season distinguishes between samples that are low disease risk versus those that are very low disease risk (Table 5). Most (86%, 85/99) of the samples that were low risk were also very low risk, which further verifies the safety of boreholes (Figure 5). While I do not advocate the 100 ml Colilert adaptation used here for regular monitoring, it highlighted several advantages of the 10 ml test in this situation. Since the limitations of this adaptation suggest that there may be false positives, the percentage of boreholes that are also very low risk may be higher. In addition to the difficulty of incubating a 100 ml test at any but ambient temperature in the rural setting, the Colilert snap-pack for the 100 ml test is several times more expensive than the 10 ml tube tests. Therefore, the additional cost and difficulty associated with these tests may not warrant the small number of additional sites that are identified with a slight increase in risk of disease. 61 Comparisons Hand-dug Wells and Boreholes. While both boreholes and hand-dug wells spanned a range of disease-risk categories, the majority of boreholes were low disease risk, while the majority of hand-dug wells were high disease risk (Figures 2 and 3). When a statistical comparison was made between samples taken from the Suhum District for the two well types, the variation was great enough to show a statistical difference in the comparison of the disease-risk category distribution for the hand-dug wells as compared with the boreholes (Figure 6). The test results using the Portable Microbiology Laboratory verified that in the Suhum District, where there are both a large number of hand-dug wells and boreholes, the boreholes are of superior quality (Figure 6). The data verified the public health policy of constructing boreholes. Continued use of the Portable Microbiology Laboratory can further verify that the substantial investment that has been made in Ghana and elsewhere to construct boreholes is warranted by the low-disease-risk level of the water in the boreholes. Boreholes by Region. There were observed variations between the Suhum and Nanumba districts that might account for the higher proportion of low disease-risk boreholes in Suhum than in Nanumba (Figure 3). The most notable variation was the different level of government attention and resources devoted to drinking water in each of the districts. Other research in Ghana, similarly, has noted lack of government policy and regulations and political neglect as factors in effective control of water contamination (3, 29). 62 The Suhum District had five full-time field officers assigned to water and sanitation and three motorbikes for transportation within the district. The field officers made regular visits to communities to meet with the village WatSan committees and, simultaneously with this research, were in the process of conducting their own survey on flow rate and village policies for collection of fees for maintenance of boreholes (Appendix B). The WatSan committees appeared well organized and were easy to locate in each community. Many communities had a local person trained in minor maintenance. At one village, the local mechanic brought out his tools and demonstrated his pride in being able to maintain the community pump. Three additional mechanics with greater skills were available to fix pumps in the district for a small fee from the funds collected by the village WatSan committee as part of its water management plan (household tax or “pay as you pump”). While the majority of households did not appear to have access to a latrine, there had been projects to provide latrines to a few households in the majority of villages (Gabriel M. Nartey, personal communication). During the period of my data collection, the Nanumba District was recovering from a conflict between the three tribes that live within the district and a large part of the governmental funds provided to the district were spent on supporting the military presence that enforces the peace, rather than other government services such as sanitation infrastructure. Additionally, the Nanumba District, by contrast with the Suhum District, had only one full-time officer and a secretary for district water and sanitation. The visual sanitation survey of boreholes in the Nanumba District identified fewer latrine 63 construction projects or households with even unimproved latrines than were reported in the Suhum District (personal observation). The minimal staff available to give attention to the maintenance of the Nanumba water sources may explain the greater proportion of higher-disease-risk boreholes in the district. In no Nanumba District village could I find a WatSan committee or anyone who self-identified as being trained in health, sanitation or pump maintenance. In some of the villages visited, I met with the Guinea worm volunteer whose responsibility was to contact the local NGO if a potential case was spotted in the village. Villagers, on occasion, expressed frustration to me in their attempts to receive outside assistance with well maintenance. It was unclear if there were skilled mechanics available to visit such villages, even if the community had funds available to pay for a mechanic. I visited one community from the minority side during the war whose well had been vandalized during the conflict. The well had the potential to be rehabilitated for far less than the cost of digging a new well. However, the villagers expressed frustration that this rehabilitation project, for whatever reason, was not carried out. Another community had its pump break down shortly before my first visit, and it was still broken three months later during my second visit. Villagers reported multiple visits by community members to the district capital to request assistance. Political neglect and lack of governmental funding may be the root causes of the poor community drinking water and sanitation infrastructure, which has been reported in other studies of nearby districts (3). The Nanumba District is one of the last remaining regions in Ghana with Guinea worm disease as an endemic health problem. Construction of boreholes in the 1960s to 64 1990s played a role in reduction of this disease (18). Today, there is a large effort that is being undertaken by the Carter Foundation to fully eradicate this debilitating waterborne parasite, which enters a human who drinks untreated surface water containing the Guinea worm larvae and causes a painful parasitic worm to erupt from the skin, usually in the lower extremities (5). My research in this district was conducted with the assistance of a staff member of the NGO that monitors for cases of Guinea worm and ensures confinement of such persons until they are recovered. Because of the large size of the Guinea worm, cloth filtration of drinking water has sometimes been advocated in regions where this disease is prevalent (31). While this has been effective in reducing cases of Guinea worm, it is not sufficient to make water safe to drink, because cloth filtration with a mesh size of 20 by 20 m will not filter out bacteria or viruses (19). The Portable Microbiology Laboratory tests can demonstrate to healthcare workers the need to respect the risk of bacterial contamination while in the process of eradicating Guinea worm. Continuing to advocate methods such as cloth filtration may lead to poor practices that are difficult to correct later. Boreholes by Season. The observation of changes in borehole water quality over time supports the public health protocol for periodic testing of drinking water. The majority (86%, 68/79) of boreholes tested in this study were low disease risk both times they were tested. The identification of a few (11) boreholes in the Nanumba District that had moderate and high disease risk confirms the need for periodic testing of each borehole, not just a sampling of a few boreholes in the region. 65 There was no statistical difference between the distribution of disease risk in the dry season and rainy season for boreholes in the Nanumba District. While four of the 32 boreholes that were tested twice showed an increase in the level of E. coli contamination from the dry to the rainy season, one borehole showed a decrease in level of E. coli contamination from the dry to the rainy season. Therefore, while this research does not indicate a greater disease risk during a particular season, it does demonstrate that five of the 32 boreholes (15%) changed in the level of E. coli contamination during the fourmonth period. Inclusion of the 100 ml data demonstrates that 10 of the 32 boreholes (31%) changed during this period. Whether or not the changes in water quality in these 10 boreholes were due to seasonal factors or to other factors, the considerable percentage that changed supports the policy of periodic testing. One possible cause of the change in contamination levels over time in the Nanumba District was the prevalence of animals at the boreholes. Large livestock near the boreholes can occur in the Nanumba District during the dry season, when the cattle herders flood the drainage channel for their cattle to use as a drinking trough (personal observation). Use of the Portable Microbiology Laboratory in combination with an inspection survey could help to sensitize the community to this disease risk. A future study could look at both the quality of water inside the well as well as the water standing in the drainage trough. Potential mitigations of this disease-risk factor exist, such as channeling water to a separate area for use by the animals. Community engagement in local health issues through a combination of using the Portable Microbiology Laboratory 66 and a routine sanitary inspection survey could raise awareness of the importance of keeping animals away from the human source of drinking water. Boreholes and Household Storage Units. Point-of-use water quality monitoring is recognized, along with safe household water storage and treatment, as a means of addressing post-collection contamination (30, 41). My study included household testing on a one-time basis. The systematic meta-analysis of earlier studies of contamination between source and point-of-use (household) by Wright et al. found that none of the studies in high bacteria count locations used E. coli as an indicator, but instead used total coliform or thermotolerant coliform (41). My study, by contrast, used E. coli as the indicator to compare contamination between source and household, which has a stronger link to public health. My study detected high coliform counts in many sources where E. coli were not present. In other studies that only looked at thermotolerant coliform levels, these non-E.coli coliform bacteria could give a false-positive indication of fecal contamination. Water from 78% (18/23) household storage units in Nanumba that were tested was either moderate or high disease risk. This was a consistently higher disease risk than the water from nearby boreholes. No household in this study had higher quality water than at the point source. Various observed circumstances may explain the higher disease risk. No household reported any use of water purification techniques, such as filtration, except for limited use of boiling. The lack of household-level purification is consistent with the same or higher level of disease risk that the test results revealed in households as compared to the nearest borehole.Considering another possible factor, Wright et al. have 67 noted that transportation methods and storage are a concern for water quality in locations where point sources are the primary source and water is not delivered directly to households through pipes (41). The drinking water that was the subject of this study was commonly transported from the point sources to the household storage units in metal or plastic bucket-shaped containers of the size that women and girls of the region can carry on their heads (personal observation). Once inside the house compound, the water was stored in wide-mouth clay or concrete pots (often with a lid) holding 100-500 liters. Water stored in such pots at the household level was used both for hygiene purposes and also as a source of drinking water. Repeated dipping of hands or a shared cup is one possible explanation for the increased contamination observed in households, as compared to nearby boreholes. Many families had multiple buckets for collecting and transporting water (personal observation). Wright et al. also note the risks of contamination during such bucket transport (41). I observed an anecdote of risky behavior myself. During one village interview in my study, a group of women all stopped and put down their buckets to participate in the testing and discussion about the household water. During this time, a small child, perhaps 18-months old, had a diarrheal episode in the midst of the group. The mother used a scrap of wood to scoop up the fecal matter and dirt and remove it a few meters off into the bush. The mother then proceeded to wash the toddler’s behind by repeatedly dipping her hand into the bucket used for transporting water from the point source to her household. No one in the group acted as if this were behavior out of the ordinary. 68 Many families reported using multiple sources of water to fill their household storage units, even if they lived adjacent to the borehole. Reasons articulated for accessing multiple sources included breakdown of the pumping mechanism, inadequate water in the well to meet all needs, seasonality of the supply, or dislike of the taste of certain water supplies (especially deep wells). The use of these multiple sources of water, as well as lack of education regarding sources of disease, as evidenced by the observed diarrheal episode, could account for the higher level of disease risk found in the household supply than in the closest borehole in 15 (68%) of the 22 cases. These results are consistent with other studies that have looked at the degradation of water quality from the point source to the household (41). Health Education. One of the goals of the CWSA is to promote proper hygiene. Even if the water supply is of high quality at the source, it can become contaminated due to poor handling and storage, which, as discussed, may account for the disease-risk results addressed in the previous section. The Portable Microbiology Laboratory can be used as part of participatory testing to trace water quality from various sources available in the community through transport, storage, household level purification, and, finally, consumption. Testing the water at various stages along this path can provide evidence to identify water sources that are moderate to very high disease risk and engage the whole community in discussing how water sources get contaminated, how to treat contaminated water to make it safe to drink, and how water sources with a low disease risk can be kept low risk. 69 In the last two years, I have led community workshops with this particular focus in Cambodia, utilizing the perspective gained from this study. Workshops have extended to information about the size of bacteria, how they grow, and germ theory of disease (28). Through this use of evidence-based microbiology to test their source and household water, community members can come to understand the health risks associated with safe water handling. In the first session of a two-day workshop, participants identify, sample and inoculate local water sources and household supplies into the two tests. Next, participants use body-incubation methods on samples from their own water source. Following the incubation period, participants meet again to interpret and discuss the results from their personal water supplies. In this way, participants have knowledge of their individual situation and can share this with their family and neighbors. Together with a discussion of the germ theory of disease, the direct observation of growth of E. coli colonies on the Petrifilm in some or all of the samples can lead to an effective discussion of possible reasons for contamination and appropriate actions to correct the problem. Point-of-use water quality monitoring is recommended along with safe household water storage and treatment as a means of addressing post-collection contamination (41). The Colilert/Petrifilm method is uniquely adapted for use directly as part of such health education programs to promote safe water handling. In this study, most of the household samples taken in the Nanumba District were inoculated either by a community member or by a child. This demonstrates that the technique for inoculating the sample can be learned and implemented quickly. Community participation also provided an opportunity 70 for discussion about water storage, sanitation, and proper maintenance of the community well. At one well that was tested in Ghana, a University of Ghana, Legon teaching assistant utilized the results to explain to children the importance of keeping the apron of the borehole clear of debris and not to abuse the pump mechanism, which causes breakdowns (Figure 9). 71 Figure 9. A University of Ghana teaching assistant talks to a group of school children about the importance of proper well maintenance. 72 Application of Findings The ease of using the Portable Microbiology Laboratory and the ability to collect and process the results rapidly by existing field staff and/or community members makes the development of a Water Safety Plan that incorporates evidence-based microbiology possible. The following sections address (1) system assessment as a baseline to set policy, (2) community-based self-monitoring and management of the local water source, and (3) verification/validation of policy and surveillance. System Assessment and Cost Considerations. The CWSA plan of testing two boreholes per district per year results in many boreholes in each district going untested, because a typical district has many boreholes. There are on average more than 100 boreholes per district (8). WHO recommends testing of all boreholes in a district once every three to five years in order to assess and validate policy (39). Assuming that the national laboratory is used, which at the time of this study charged $25 per sample for a thermotolerant coliform test, the current cost per district is $50 per year to test two boreholes (Joseph Ampofo, Water Research Institute, Ghana, personal communication). The cost of using the Portable Microbiology Laboratory is dramatically less, approximately $2 per test. Without increasing the district cost, 25 boreholes could be tested for E. coli using the 10 ml Colilert P/A test. For the average district, this frequency of borehole testing would be in accord with the WHO recommendation of testing all boreholes once every three to five years. This would be a definite improvement over the current testing regime. 73 Transportation costs associated with testing in Ghana can also be reduced in shifting away from the thermotolerant coliform test to the Portable Microbiology Laboratory. The cost of transporting samples back to a central laboratory can be avoided. The Colilert ingredients are premeasured as a shelf-stable dry powder into marked tubes and can be distributed and stored for use in rural Ghana without refrigeration. The Petrifilm test, too, is shelf stable and acceptable for distribution to rural areas. Unused Petrifilm tests need to be stored in a resealed foil packet to prevent moisture damage; this can be done with masking tape. Moreover, the testing in the field that is possible with the Portable Microbiology Laboratory eliminates false negative results that may occur from prolonged transport to a laboratory and delay in laboratory processing of the sample (6). In summary, the Portable Microbiology Laboratory tests obtain better, more timely data than the thermotolerant tests and at a lower cost. Plus, the results should be more intelligible to the community, because of their timeliness and their graphical clarity. That is, for example, a fluorescent blue color in the Colilert tube or a Petrifilm containing blue colonies with gas from an on-the-spot test visually indicates the presence of E. coli and an unsafe water source (intolerable disease-risk level). By comparison, a written lab report, received a week or more later from a central laboratory of 50-500 thermotolerant coliform per 100 ml, requires further interpretation. Acceptance of the Portable Microbiology Laboratory. The Portable Microbiology Laboratory is a kit that complements the current monitoring practices of the CWSA. Biological testing is easily combined with other elements of pump maintenance and testing. For example, District Water and Sanitation Team member 74 Gabriel M. Nartey was able to measure the depth of water in the borehole simultaneously to my inoculating the Colilert sample, and to the area mechanic and District Water and Sanitation Team member Gershon Hiamadey recording information about the pump flow rate (Figure 10). Portable Microbiology Laboratory supplies for an entire day fit in a small twoliter bag that easily can be carried while riding the standard issue CWSA motorbikes used to visit the communities for other monitoring. The ease of body incubation of the multiple samples taken each day eliminates the need for a bulky incubator powered by the electricity that is scarce in rural Ghana. In my study, the ease of body incubation attracted community volunteers in Ghana who participated in inoculation of the samples, a similar circumstance to subsequent trials by the author in Cambodia, and to earlier trials in Kenya and Tanzania (24, 25, 26). 75 Figure 10. The District Water and Sanitation Team can simultaneously take a variety of measurements and perform maintenance on a borehole. From left to right, Gabriel M. Nartey measures the depth of water in the borehole, Katherine Parker (kneeling) inoculates the Colilert sample, and the area mechanic assists Gershon Hiamadey record information about the pump flow rate. Women with water collection buckets in background wait patiently. 76 Integration of Portable Microbiology Laboratory into Existing Surveillance. WHO recommends the use of microbiological testing in conjunction with a sanitation inspection survey to identify other disease-risk factors (39). In Ghana, though, the district officers do not always have the ability to collect water samples and transport them to the few regional laboratories in a safe and efficient manner that would allow for laboratorybased microbiological testing (personal observation). Therefore, up until now, it appears that regular microbiological testing has not been included in surveys, because it is seemingly impractical in terms of time and cost to conduct. However, this research demonstrated that the Portable Microbiology Laboratory field kit for microbial testing could be used in conjunction with other assessment surveys. In the Eastern Region of Ghana, which includes the Suhum District, the District Water and Sanitation Team currently visits all communities to conduct a management survey of the borehole, monitor the flow rate, and maintain the pump, as part of its surveillance of point source water supplies. The team members who accompanied me rapidly learned the techniques involved to ensure sterile, microbiological quality and were soon instructing members of the WatSan committee and even local children in how to perform the tests. Community-based Monitoring. The results presented here indicate that periodic community-based testing of boreholes and hand-dug wells may be an appropriate level of monitoring for this type of water source in Ghana. The Portable Microbiology Laboratory is particularly suited to this because the community members themselves can perform the tests. Having test results available to a local community with hand-dug wells creates 77 some options about actions that can be taken to provide low disease-risk water pending the construction of boreholes. If an unacceptable risk level is identified with the Portable Microbiology Laboratory, even with a borehole, immediate action can be taken. For example, during my testing, when I identified one high disease-risk borehole, I was able to provide chlorine tablets to the local contact for him to take back to the community for an immediate shock chlorination treatment. Once the community has done an initial assessment of all point sources with both the Colilert and Petrifilm tests and identified all of the high and very high disease-risk sources, it is not necessary to use ongoing monitoring for these high disease-risk sources. Where possible, alternative moderate- or low-disease-risk sources should be identified for use, or appropriate household-level purification and safe storage techniques should be applied. For sources that are generally low disease risk, such as the boreholes and some of the hand-dug wells with pumps tested in this study, ongoing monitoring by the WatSan committee using only the Colilert test has potential advantages to the community. Based on the data collected, the community could be assured that the source was consistently low disease risk. After confirmation by follow-up testing that any 10 ml Colilert sample that was positive for E. coli were a true problem rather than an anomaly, 1 ml Petrifilm tests could be used to determine the extent of the contamination and if significant degradation to the well or borehole existed such that alternative sources should be sought or household treatment like boil orders or other appropriate purification should be implemented for water from this source for a period of time. In line with the CWSA 78 policy of shifting responsibility of monitoring the water supply from the CWSA to the community, the Portable Microbiology Laboratory gives a low cost option for the communities to take greater responsibility for water safety. Validation of CWSA Policy. This study shows that the Portable Microbiology Laboratory can be used to verify the policies set by the CWSA to focus on construction of boreholes. For water systems that receive regular monitoring, WHO presents a grading system for performance targets based on the percentage of samples from the system classified to be below a tolerable risk level (typically negative for E. coli at 100 ml where water is chlorinated) (39). WHO notes that developing countries may need to set intermediate tolerable disease-risk levels during the period when the primary focus is on improving access, such as low disease risk (negative for E. coli at 10 ml or the pre-1989 standard in the USA of negative for E. coli at 50 ml) (39). The 2001 WHO Guidelines lay out clearly that no E. coli in 10 ml of water constitutes a low disease risk and thus is an appropriate tolerable risk level (37). All boreholes in the Suhum District met this standard, and the district performance can be rated as excellent. Seventy-six percent (76%, 35/46) of the boreholes in Nanumba met this standard, and the district performance can be rated as moderate. The relatively low cost of testing associated with the Portable Microbiology Laboratory, about $2/test, enables not just water systems but also point sources to be tested more frequently. Frequent testing can allow for immediate identification of problem sources for urgent action and also for standards to be set for individual point sources. In addition to providing sufficient data so that, for the first time, the CWSA can 79 have an overall grade for different districts, the results presented in this thesis also identified a high-disease-risk source for urgent action. Use of the combination of tests in the Portable Microbiology Laboratory could transform water testing in Ghana and bring its testing regime into substantial accord with WHO testing recommendations (38). The portable testing provides simple, inexpensive, and accurate results that correspond to the WHO disease-risk categories. The results of this study identified water sources in all four WHO disease-risk categories of low, moderate, high, and very high, indicating sensitivity of testing that corresponds to current contamination levels. The testing can be used both to provide a baseline to set national policies and strategies and for ongoing verification of quality. Furthermore, by involving communities in the testing and interpretation of these results, progress can be made in reaching goals for health education and improving domestic hygiene. The tests are commercially available and could be integrated in Ghana into current water supply surveillance practices to improve public health. 80 APPENDICES 81 APPENDIX A Management of the Rural Water Supply in Ghana The Community Water and Sanitation Agency (CWSA) is currently part of the Ghana Ministry of Works and Housing. In 1994, the National Community Water and Sanitation Program was launched as a decentralized support structure to the District Assemblies (area government units) in the provision of potable water and related sanitation services to rural communities and small towns in Ghana. Later, by Parliamentary Act 564 in December 1998, the CWSA was formally split from the Ghana Water and Sewage Corporation (more recently known as the Ghana Water Company Limited) and organized within the Ministry of Works and Housing (9). The CWSA mandate is to the rural communities and small towns that account for about 70% of the population of the country. The head office for the CWSA is located in Accra. The CWSA functions in all ten administrative regions of Ghana through regional offices. The Regional Water and Sanitation Teams provide support to the District Assemblies to form and train District Water and Sanitation Teams. CWSA operates in 128 districts and in 13,488 communities (9). External Support Agencies. In the urban sector, there has been a move towards privatization of water. Privatization started with "structural adjustment" programs initiated in 1983 and culminated in the introduction of foreign management support to the Ghana Water Company Limited in 2006 (23). In the rural sector, by contrast, potential profit margins for private investors are low. This is because there is a high cost to the 82 necessary investment and because low rural income levels require low water rates to be charged (23). Provisioning of water to this sector has therefore become the responsibility of the rural communities themselves, with financial and technical assistance from “external support agencies,” which are foreign governments and non-governmental organizations (NGOs) that provide aid (23). The key role of such external support agencies is reflected in the country's national program for Community Ownership and Management of the water and sanitation facilities. This program seeks to integrate the assistance from external support agencies into building and maintaining locallycontrolled rural water supplies, which is different from the earlier approach of principal reliance on the national Ghana Water and Sewerage Corporation (9). CWSA Mandate. The specific function of the CWSA in the country's organizational structure for providing rural water and sanitation service, as set forth in Act 564, is to (i) promote the sustainability of a safe water supply and related sanitation services in rural communities and small towns; and (ii) encourage the active involvement of communities, especially women, in the design, planning, construction and community management of water and sanitation projects. The CWSA is responsible for formulating strategies for the mobilization of resources, providing technical assistance, and coordinating with NGOs engaged in the development of water and sanitation projects and hygiene education. It is also the role of the CWSA to “prescribe standards and guidelines for safe water supply and provision of related sanitation services in rural communities and small towns and support the District Assemblies to ensure compliance by the suppliers of the services” (9). 83 The CWSA outlines its four goals for the minimum basic service of a water supply as: (1) it is protected year-round; (2) it provides 20 liters per capita per day; (3) it is within 500 meters of families in the community, and (4) in the case of a borehole with a hand-pump, it serves no more than 300 persons per outlet (9). Village, Community and District Roles. Implementing the national policy for Community Ownership and Management of water resources, the basic unit of the community water supply program is the village Water and Sanitation (WatSan) committee. Every village is required to have a WatSan committee with a constitution and a chairperson, treasurer, secretary, hygiene officer, and caretaker. At least one leader must be a woman; a woman as the hygiene officer typically fulfills this requirement (23). The WatSan committee is required to contribute towards the capital cost of its facility at the rate of 5% for communities and 2.5% for small towns and to pay the full operation, maintenance, and repair cost (8). At the time of this study, the 5% share for a local community came to $350 for a typical $7000 borehole. The fees that are intended to cover these capital and operational costs are typically collected either through a monthly tariff on each household, monthly tariff per person above 18, or through a “pay as you fetch” tariff per bucket of water collected (Appendix B). The District Water Sanitation Team directly supports the village WatSan. The Team helps to coordinate additional services for the WatSan committee that are provided through the private sector and external support agencies. These include prioritizing the distribution of external support agency funding for the building of new water sources, monitoring and interfacing with private contractors engaged in construction, and setting 84 guidelines on rates to be charged by the area mechanic (Gabriel M. Nartey, personal communication). Constructing Water Projects. In recent years, there has been a significant effort at construction of new boreholes. WHO and UNICEF report the percentage of the rural population having access to improved sources of drinking water in 2008 as having increased to 3% piped on premises, 71% other improved sources (boreholes and handdug wells), and 26% with access only to unimproved sources (40). For example, in the Eastern Region of Ghana, which is part of the study area here addressed, there have been five construction phases for boreholes supported by various external support agencies. These phases were "3000 Wells" (1978-1984) for 576 boreholes, "JICA" (1997-1999) for 329 boreholes, "DANIDA I" (1999-2003) for 477 boreholes, "RWSP III" (2002-2005) for 200 boreholes, and "DANIDA II" (2004-2008) for 32 boreholes. There was also a period of rehabilitation of boreholes from 1997-2000. (Theo Mensa, Eastern Region Water and Sanitation Team engineer, personal communication) Since 1994, as part of the National Community Water and Sanitation Program, there has been new construction of 524 small-town pipe systems, 15,654 boreholes and 1,430 hand-dug wells (9). This brings the national coverage for potable water supply in both rural communities and small towns in the country up to 51.7%, 5% higher than two years previously (9). The CWSA plans to increase this coverage from 74% to 85% by 2015. The CWSA is currently developing a plan for regular monitoring of the quality in small town pipe systems and random monitoring of quality in point sources. While the plan includes regular monitoring of the pipe systems, the current policy is that each 85 District Assembly is responsible for testing two randomly selected boreholes each year (Charlotte Engmann, National CWSA engineer, personal communication). Also, since 1994, sanitation in Ghana’s rural areas has been addressed through the construction of 2,184 institutional "KVIP" latrines and 42,588 household latrines (8). Percentage of the rural population having access to sanitation facilities in 2008 stands at 38% access to shared facilities, 7% access to improved (KVIP) latrines at household level, and 21% access to unimproved facilities (household latrines), with the remaining 34% having only open defecation options (40). 86 APPENDIX B Water Facilities Monitoring Sheet for WatSans 87 88 89 APPENDIX C Survey of Point-Source Water Supply 90 91 LITERATURE CITED 1. Allen, M.J., Payment, P., & Clancy, J.L. (2010). Rapid microbial methods can improve public health protection. Journal of American Water Works Association, 102:44-51. 2. Ampofo, J.A. (1997). A survey of microbial pollution of rural domestic water supply in Ghana. International Journal of Environmental Health Research, 7:121130. 3. Atipoka, F.A. (2009). Water supply challenges in rural Ghana. Desalination, 248:212-217. 4. Boschi-Pinto, C., Velebit, L., & Shibuya, K. (2008). Estimating child mortality due to diarrhoea in developing countries. Bulletin of the World Health Organization, 86:710–717. 5. CDC. Dracunculiasis. Accessed on April 25, 2011, from http://www.dpd.cdc.gov/dpdx/HTML/Dracunculiasis.htm 6. Chao, W.L. (2006). Evaluation of Colilert-18 for the detection of coliforms and Escherichia coli in tropical fresh water. Letters in Applied Microbiology, 42:115120. 7. Curiale, M.S., Sons, T., McIver, D., McAllister, J.S., Halsey, B., et al. (1991). Dry rehydratable film for enumeration of total coliforms and Escherichia coli in foods: collaborative study. Journal of Association of Official Analytical Chemists, 74:635-648. 8. CWSA. (n.d.). National Community Water and Sanitation Programme (NCWSP): A Decade of Success Story, 1994-2004 pamphlet. Accra: Community Water and Sanitation Agency. 9. CWSA. (n.d.). National Community Water and Sanitation Programme brochure. Accra: Community Water and Sanitation Agency. 10. DuFuor, A., Snozzi, M., Koster, W., Bartram, J., Ronchi, E., et al. (Eds.). (2003). Assessing Microbial Safety of Drinking Water. London: IWA Publishing. 92 11. Edberg, S., Allen, M.J., Smith, B.D., & the National Collaborative Study. (1988). National field evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and Escherichia coli from drinking water: comparison with presence-absence techniques. Applied and Environmental Microbiology, 54:1003-1008. 12. Edberg, S., Rice, E., Karlin, K., & Allen, M. (2000). Escherichia coli: the best biological drinking water indicator for public health protection. Journal of Applied Microbiology, 68:106-116. 13. Feng, P.C.S., & Hartman, P.A. (1982). Fluorogenic assays for immediate confirmation of Escherichia coli. Applied and Environmental Microbiology, 43:1320-1329. 14. Fewtrell, L., & Bartram, J. (2001). Water Quality: Guidelines, Standards and Health. London: IWA Publishing. 15. Gangar, V., Curiale, M.S., Lindberg, K., & Gambrel-Lenarz, S. (1999). Dry rehydratable film method for enumerating confirmed Escherichia coli in poultry, meats, and seafood: collaborative study. Journal of AOAC International, 82:7378. 16. Howard, G. (2002). Water Quality Surveillance: A Practical Guide. Leicestershire: WEDC, Loughborough University. 17. Howard, G., & Bartram, J. (2003). Domestic Water Quantity, Service Level and Health. Geneva: World Health Organization. 18. Hunter, J.M. (1997). Boreholes and the vanishing of Guinea worm disease in Ghana’s upper region. Social Science Medicine, 45:71-89. 19. Huq, A., Yunus, M., Sohel, S.S., Bhuiya, A., Emch, M., et al. (2010). Simple sari cloth filtration of water is sustainable and continues to protect villagers from cholera in Matlab, Bangladesh. mBio, 1:e00034-10. 20. IDEXX Laboratories. Accessed on April 5, 2011, from http://www.idexx.com/view/xhtml/en_us/water/colilert.jsf 21. Koehler, K.J., & Larntz, K. (1980). An empirical investigation of goodness-of-fit statistics for sparse multinomials. Journal of the American Statistical Association, 75:336-344. 22. Leclerc, H., Mossel, D.A., Edberg, S.C., & Struijk, C.B. (2001). Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Annual Review of Microbiology, 55:201-234. 93 23. Mensah, K. (1998). Restructuring the delivery of clean water to rural communities in Ghana: the institutional and regulatory issue. Water Policy, 1:383-395. 24. Metcalf, R.H., & Williams, E.F. (2001). Use of Colilert and E. coli Count Petrifilms to assess water quality in rural Tanzania, abstr. Q-327. Abstr. 101st Gen. Meet. Am. Soc. Microbiol. (p. 650). Washington, DC: American Society for Microbiology. 25. Metcalf, R.H., Polinelli, C., Kipesha, F.R., & Swedi, S. (2003). Use of Colilert and E. coli Count Petrifilm for point source water testing in Dar es Salaam, Tanzania, abstr. Q-422. Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. (p. 590). Washington, DC: American Society for Microbiology. 26. Metcalf, R.H., & Polinelli, C. (2005). Transforming point source water testing in Kenya, abstr. Q-329. 105th Gen. Meet. Am. Soc. Microbiol. Washington, DC: American Society for Microbiology. 27. Metcalf, R., & Polinelli, C. (2010). Bringing water microbiology to the community level in developing countries, abstr. 3322A. World Water Congress. London: International Water Association. 28. Metcalf, R.H., & Stordal, L.O. (2010). A practical method for rapid assessment of the bacterial quality of water. Nairobi: UN-Habitat, UNON Publishing Services Section. 29. Obiri-Danso, C.A., Weobong, C.A., & Jones, K. (2005). Aspects of health-related microbiology of the Subin, an urban river in Kumasi, Ghana. Journal of Water and Health, 3:69-76. 30. Peter, K.J., Ensink, H.J., Jayasinghe, G., van der Hoek, W., Cairncross, S., et al. (2002). Domestic transmission routes of pathogens: the problem of in-house contamination of drinking water during storage in developing countries. Tropical Medicine and International Health, 7:604-609. 31. Reuters (Australian Broadcasting Corporation). Fighting the 'fiery serpent' in Sudan. Accessed on June 6, 2008, from http://www.abc.net.au/news/stories/2008/06/06/2267262.htm 32. Rottier, E., & Ince, M. (2003). Controlling and Preventing Disease: The Role of Water and Environmental Sanitation Interventions. Leicestershire: WEDC, Loughborough University. 33. Schraft, H., & Watterworth, L.A. (2005). Enumeration of heterotrophs, fecal coliforms and Escherichia coli in water: comparison of 3M Petrifilm plates with standard plating procedures. Journal of Microbiological Methods, 60:335-342. 94 34. Sobsey, M.D. (2001). Microbial detection: implications for exposure, health effects, and control. In G. Craun (Ed.), Safety of Water Disinfection: Balancing Chemical and Microbial Risks (2nd Ed.). Washington, DC: ILSI Press. 35. Standridge, J. (2008). E. coli as a public health indicator of drinking water quality. Journal of American Water Works Association, 100:65-75. 36. Vail, J., Morgan, R., Merino, C., Gonzales, F., Miller, R., et al. (2003). Enumeration of waterborne Escherichia coli with Petrifilm plates: comparison to standard methods. Journal of Environmental Quality, 32:368-373. 37. WHO. (2001). Guidelines for Drinking Water Quality (2nd Edition). Geneva: World Health Organization. 38. WHO. (2005). Water Safety Plans: Managing Drinking-water Quality from Catchment to Consumer. (Davison, A., Howard, G., Stevens, M., Callan, P., Fewtrell L., et al., Eds.). Geneva: World Health Organization. 39. WHO. (2008). Guidelines for Drinking Water Quality, Incorporating 1st and 2nd Addenda, Vol. 1, Recommendations (3rd Edition). Geneva: World Health Organization. 40. WHO and UNICEF. (2010). Progress on Sanitation and Drinking-water: 2010 Update. Geneva: World Health Organization. 41. Wright, J., Gundry, S., & Conroy, R. (2004). Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Tropical Medicine and International Health, 9:106-117. LAWS AND REGULATIONS CITED 42. 40 C.F.R. § 141 (2011)