How to carry out a titration
advertisement
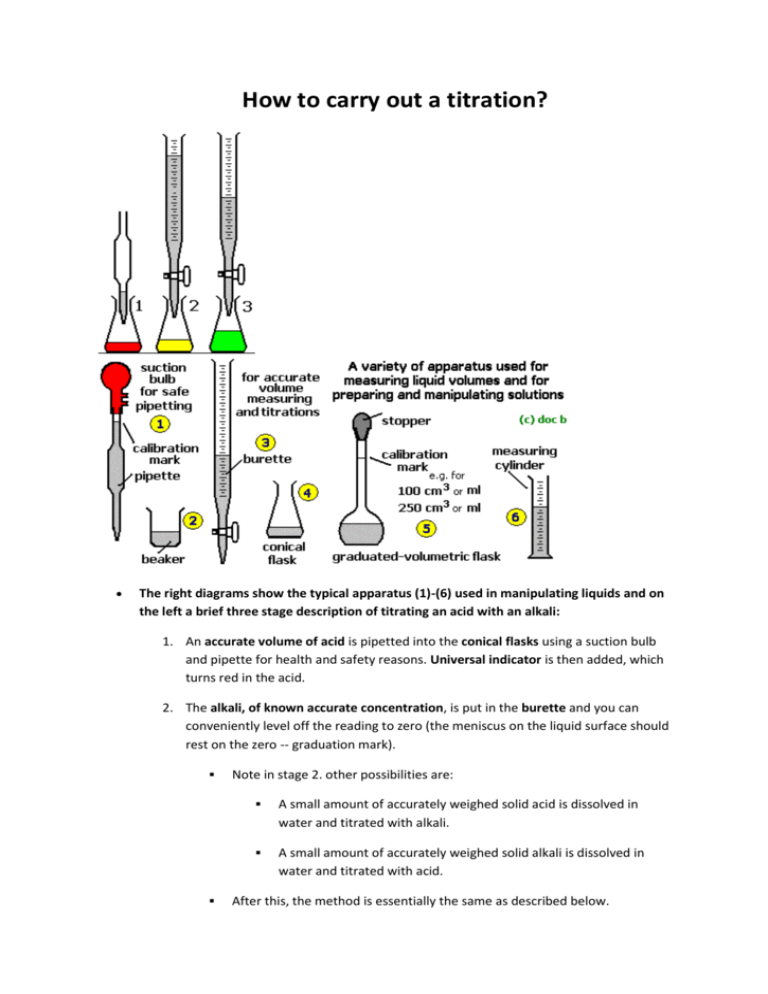
How to carry out a titration? The right diagrams show the typical apparatus (1)-(6) used in manipulating liquids and on the left a brief three stage description of titrating an acid with an alkali: 1. An accurate volume of acid is pipetted into the conical flasks using a suction bulb and pipette for health and safety reasons. Universal indicator is then added, which turns red in the acid. 2. The alkali, of known accurate concentration, is put in the burette and you can conveniently level off the reading to zero (the meniscus on the liquid surface should rest on the zero -- graduation mark). Note in stage 2. other possibilities are: A small amount of accurately weighed solid acid is dissolved in water and titrated with alkali. A small amount of accurately weighed solid alkali is dissolved in water and titrated with acid. After this, the method is essentially the same as described below. 3. The alkali is then carefully added by running it out of the burette in small quantities, controlling the flow with the tap, until the indicator seems to be going yellow-pale green. The conical flask should be carefully swirled after each addition of alkali to ensure all the alkali reacts. 4. Near the end of the titration, the alkali should added drop-wise until the universal indicator goes green. This is called the end-point of the titration and the green means that all the acid has been neutralised. The volume of alkali needed to titrate-neutralise the acid is read off from burette scale, again reading the volume value on the underside of the meniscus. The calculation can then be done to work out the concentration of the alkali. 5. Universal indicator, and most other acid-base indicators, work for strong acid and alkali titrations, but universal indicator is a somewhat crude indicator for other acidalkali titrations because it gives such a range of colours for different pH's. Examples of more accurate and 'specialised' indicators are: titrating a strong alkali with a strong acid (or vice versa): e.g. for sodium hydroxide (NaOH) - hydrochloric/sulphuric acid (HCl/H2SO4) titrations, use ... phenolphthalein indicator (pink in alkali, colourless in acid-neutral solutions), the end-point is the pink <==> colourless change. Litmus works too, the end point is the red <==> purple/blue colour change. titrating a weak alkali with a strong acid: e.g. for titrating ammonia (NH3) with hydrochloric/sulfuric acid (HCl/H2SO4), use ... methyl orange indicator (red in acid, yellowish-orange in neutralacid), the end-point is an 'orange' colour, not easy to see accurately. screened methyl orange indicator is a slightly different dye-indicator mixture that is reckoned to be easier to see than methyl orange, the end-point is a sort of 'greyish orange', but still not easy to do accurately. titrating a weak acid with a strong alkali: e.g. for titrating ethanoic acid (CH3COOH) with sodium hydroxide (NaOH), use ... phenolphthalein indicator (pink in alkali, colourless in acid-neutral solutions, pink in alkali), the end-point is the first permanent pink. methyl red indicator (red in acid, yellow in neutral-alkaline), the end-point is 'orange'. titrating a weak acid with a weak alkali (or vice versa): These are NOT practical titrations because the pH changes at the end-point are not great enough to give a sharp colour change with any indicator.