Experiment – Decomposition CFCs break down in the stratosphere
advertisement

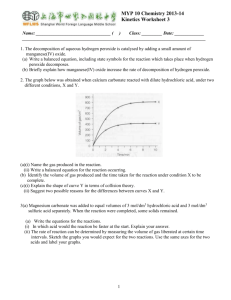
Experiment – Decomposition CFCs break down in the stratosphere and destroy ozone molecules through a process known as decomposition. Decomposition reactions occur when one reactant breaks down to form two or more new substances. The general equation for a decomposition reaction is: XY X + Y The CFC dichlorodifluoromethane gas (CCl2F2) decomposes with the help of UV light. The UV light acts as a catalyst to speed up the reaction. The reaction produces highly reactive chlorine radicals (Cl) and chlorodifluoromethane gas (CClF2) and is called a catalytic decomposition reaction. This reaction is shown below. dichlorodifluoromethane gas (+ UV light) chlorine radicals + chlorodifluoromethane gas CCl2F2 CClF2+ Cl These free radicals then react with ozone molecules in a single displacement reaction – in this reaction one element in a compound replaces another in a compound. The general formula is: A + BX AX + B The chlorine radicals (Cl) displace an oxygen atom from ozone (O3) to form hypochlorite (ClO) and oxygen gas (O2). Chlorine radicals + ozone hypochlorite + oxygen gas Cl + O3 ClO+ O2 The hypochlorite can then react with other chemical compounds and reform as a chlorine radical. These radicals can then continue to attack thousands more ozone molecules. This is called a chain reaction – the products of the reaction promote the spread of the reaction. Pre Lab Activity: Answer the following questions 1. Define the term “decomposition reaction” and give the general formula. 2. Define the term “catalyst”. Explain why the decomposition reaction of CFCs is called “catalytic decomposition”. 3. Define the term “chain reaction”. Explain who the destruction of ozone by CFCs is a chain reaction. 4. Define the term “single displacement reaction”. Experiment – Decomposition. Aim: To compare the decomposition of various metal carbonates Equipment: Goggles Test tubes Right angled delivery tube Spatula Bunsen burner Clamp and retort stand Limewater Matches Approx 2 grams of: o Copper carbonate o Sodium carbonate o Zinc carbonate Method: 1. Put a large measure of copper carbonate into a test tube, 2. Fit a delivery tube (with stopper) to the test tube and place in the clamp, ensuring that the delivery tube dips into a second test tube containing 3mL of limewater. See diagram below. 3. Using the Bunsen burner, gently heat the solid at first, and then more strongly. Record any observations. 4. Before turning of the Bunsen burner and stopping the heating process, remove the delivery tube from the limewater. This is to avoid “suck-back”. 5. Record all your observation in the table provided. 6. Using new test tubes, repeat steps 1-6 for both other metal carbonate samples. NOTE: if a gas is produced during this procedure, you will see bubbles passing through the limewater. If carbon dioxide is produced, the limewater will turn milky white. Safety (Risk assessment): Complete the following table, identifying the risks associated with the above experiment and the safety precautions you will be taking to manage these risks. Table 1. Risk Assessment Potential Hazards Standard Handling Procedures Results: Complete the following table. Carbonate Sodium carbonate Zinc carbonate Copper carbonate Colour before heating Table 2. Results Colour after heating Gas produced? Ease of decomposition Questions: 1. How do you know a chemical reaction has occurred during this experiment? 2. This experiment involved a reaction known as thermal decomposition. Explain what this term means. 3. Predict if the mass of the new substance left in the test tube after heating would be greater or less than the mass of the carbonate at the start of the reaction. 4. Explain why limewater as used in this experiment.