Lab sheet – SA to vol ratio
advertisement

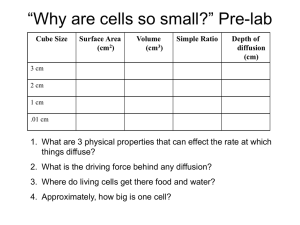
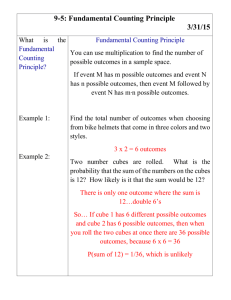
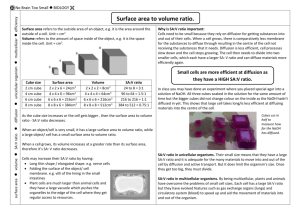
Surface area to Volume ratio Aim: To model/investigate: The change in the surface area to volume ratio with ‘cell’ size. The effect of a change in the surface area to volume ratio on the rate of diffusion. Materials: Agar-phenolphthalein-sodium hydroxide cubes (gelatin blocks) 0.1M hydrochloric acid (approximately 150mL) 30cm ruler Scalpel Paper towels 250mL beaker 100mL beaker Plastic spoon stopwatch Introduction/Background: Understanding the relationship between the size of cells (volume) and their surface area helps us to realise how the physical limitation of the size of cells is important if they are to function effectively. In order to carry out necessary chemical activities (metabolism) cells need to absorb substances that they require from their surroundings. This uptake is often dependent on the process of diffusion. Diffusion is a slow process and so to be more effective, the number of molecules that can be taken in a particular space of time should be increased. The hypothesis to be tested is that by increasing the surface area over which uptake occurs (for an object of a particular volume), diffusion can be increased and molecules can reach the centre of an object sooner than if the object has a lower surface area to volume ratio. Therefore, although the rate of diffusion is still the same, by increasing the amount of diffusion occurring, the overall rate of movement increases. This investigation makes use of a model to investigate the effect of the surface area to volume ratio on cellular diffusion. Cells of different sizes are represented by gelatin cubes, a simplification to try to increase understanding of the concept and to allow predictions to be made. When interpreting the behaviour of substances in the model (in this instance, the movement of the chemicals by diffusion) the limitations of the model must be taken into account. For example one limitation is that the jelly cubes do not have a selectively permeable boundary such as a cell membrane. The validity of the model should also be assessed. For example, the lack of a cell membrane and an energy source suggests that no active transport can take place, indicating that only diffusion is being investigated. Safety: Phenolphthalein can stain clothing. Wear safety glasses and take care not to get HCl on skin or in eyes. If acid comes in contact with any part of the body irrigate well with water. Turn the gelatin blocks with a spoon and do not handle the chemicals. Procedures: 1. Cut agar cubes into sizes of 1cm, 2cm and 3cm using a scalpel or razor blade. 2. Calculate the surface are to volume ratio of each of the cubes (see table below). Assume the cubes have exactly 3cm, 2cm or 1cm sides. Due to the indicator (phenolphthalein) and the presence of the base (NaOH, sodium hydroxide) in the cubes they will appear pink. 3. Place one of each size cube (1cm³, 2cm³ and 3cm³) into each 250mL beaker making sure that each cube is not touching the others. 4. Cover the cubes with the hydrochloric acid and leave for 10 minutes. Turn the cubes over every 2 minutes with a plastic spoon. 5. Remove the cubes from the acid after 10 minutes, quickly blotting dry the surface of the cubes using paper towel. 6. Cut each cube into 2 using the scalpel blade. Measure the depth of penetration of the acid into each cube in millimetres and record it in the table below. 7. Complete the remaining calculations in the table, determining the volume of each cube that remains coloured and calculating the percentage penetration in the last column as a measure of the proportion of the cube affected by diffusion, in cubes of different sizes. Results: Table 1: Investigating the effects of changing the surface area to volume ratio on the percentage acid penetration in agar blocks of NaOH and phenolphthalein indicator when placed into HCl. Cube size l (mm ± 0.5mm Surface area SA (6xl²) mm² Volume V (l³)mm³ 𝑆𝐴 𝑉 = 𝑆𝐴: 𝑉 Trial 1 2 10 3 4 1 2 20 3 4 1 2 30 3 4 Volume left Depth of penetration coloured 𝑉𝑐 =(l-2x)³ (x) mm % left coloured 𝑉 ( 𝑐)x100 𝑉 % acid penetration = 100-𝑉𝑐 % Average % acid penetration Standard deviation Discussion: 1. 2. 3. 4. Identify the trend in the change in surface area to volume ratio as the volume of the cube increases. Assess the accuracy of any measurements and calculations made and their relative importance in this practical. Describe the evidence that indicates that diffusion in the cube has occurred. Identify which cube (cell) showed the greatest depth of penetration of acid and which showed the least. Was this what you would have expected? Explain. 5. Explain why it was necessary to calculate the percentage acid penetration for each cube. 6. When a cell divides, it produces two identical cells that at first, are half the size of the original cell. Describe the change to the: a. Total surface area: _________________________________________________ b. Total volume: ____________________________________________________ c. Surface area to volume ratio for each cell: ________________________________________________________________ 7. These cells will then grow, but to a limited size. Clarify the significance of the results of this investigation for multicellular living organisms. 8. Discuss the limitations of the jelly cubes representing cells (hint: see Intro/background)