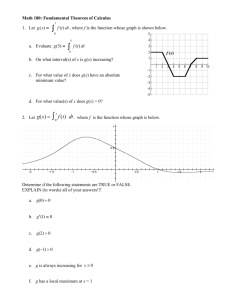
Supplemental Material for Hellmann`s Model and G(r) Hellmann`s

Supplemental Material for Hellmann’s Model and G(r)
Hellmann’s first account of chemical bonding appeared in 1933.
1
This paper showed that
Hellmann advanced an “anschaulich” (simple, easy to grasp) view of the covalent bond that introduces the notion of a decrease in the kinetic energy, an approach that he ‘supersedes’ with the ‘true quantum mechanical problem using Schrödinger’s equation’. Following on his rewriting of Fock’s version of the virial theorem, Hellmann arrives at Slater’s expression of the virial thoeorem T = -E - R(dE/dR), derived by Slater in the same year in his original derivation of the theorem.
2
Unlike Hellmann, Slater gives a full discussion of this statement by using it in conjunction with empirical potential energy curves to establish the behaviour of E, T and V versus R. Slater’s 1933 paper is the landmark paper that established the quantum mechanical explanation of chemical bonding.
Hellmann’s model of the decrease in the kinetic energy is developed in his book in discussions spread over many sections, using a number of models.
3
It is important to recognize from the outset that
Hellmann held the virial theorem in high regard as seen from a statement on page 16 coming in a section where he discusses possible functional forms for the kinetic energy: “We can select this function so that at equilibrium, the virial theorem is correct. This is necessary because the non-fulfillment of the virial theorem sharply contradicts the main equations of mechanics.” This is followed in the section dealing with the “Visual treatment of chemical forces” with the statement: “The virial theorem provides an opportunity to get a rigorously well-grounded answer to the question about the make-up of the total energy and allows one to check out mental pictures.” Any suggestion that Hellmann relegated the virial theorem to a secondary position relative any of the models that he proposed are ill-founded.
He uses the Thomas-Fermi (T-F) model along with the particle in a box model to discuss the behaviour of the kinetic energy on bonding
3;3
presenting a proof, pages 41-42, that the virial theorem is valid for the T-F model. On pages 50 -51 he uses the electron in a box model to considers the approach of two identical atoms with a single valence electron each initially in its own box. He begins by arguing that within the Thomas-Fermi model, the electron’s momentum is related to its available volume. In forming the molecule, the boxes become one and the electrostatic attraction of each valence electron with the field of the other atom gives attraction. (From this point on, the contribution arising from the change in the potential energy of the electrons is ignored, a point that, as described below, is later rectified.) In forming the molecule, that is, in joining the boxes, he argues that the size of the potential well available to each electron is approximately doubled and their kinetic energy decreases. This assumed ‘doubling of the box size’ is the basis for his model of a decrease in the kinetic energy on bonding, an assumption discredited in the text of the paper.
As noted by Hellmann, his model has ignored the contribution form the potential energy, a point he later rectifies (pages 47 -51) in a discussion of the potential energy component of the particle in a box model: “If the potential sides have a finite height, then a portion of the charge is located in the region of high potential and their density is exponentially decaying away. We are no longer convinced that the actual cause of the bonding is the decrease in the kinetic energy, since a portion of the charge has a
potential energy that can decrease upon the approach of the atoms.” a conclusion that reflects his stated belief in the virial theorem.
There are many errors associated with the view of bonding attributed to Hellmann, a number of them exemplified in a paper published in Angewandte Chemie:
4
“The reproach that Hellmann had not taken the virial theorem into account is the more grotesque is that in the same article in which he proposed the picture of the chemical bond that is named after him, Hellmann derived the virial theorem for molecules (simultaneously with and independently of Slater) and discussed in detail its role in connection with models of the chemical bond.” Firstly, Hellmann did not give a derivation of the virial theorem. He began with a statement already derived by Fock in 1932, failing, in addition, to give a detailed account of its role in the chemical bond. His work is not to be compared with Slater’s 1933 paper which not only provides an original derivation of the virial theorem but, unlike Hellmann, gives a full account of its role in chemical bonding. Secondly, he does not discuss his bonding model at any length in the 1933 paper, the first full discussion being given in his 1937 book. Thirdly, Hellmann was not opposed to admitting the role of the virial theorem in bonding, discussing in his book that he obtains his model by ignoring the contribution from the potential energy, and it thus does not fully account for bonding as it “ignores the virial theorem”. Hellmann did derive the electrostatic theorem in 1933 before repeating it in his book. However, neither in the paper nor in the book does he make any use of the theorem in a discussion of chemical bonding nor allude to its relevance in bonding, unlike Feynman’s paper that is entitled “Forces in Molecules”.
5
A disparaging comment on Slater’s discussion of bonding has recently appeared in the literature: 6 “
Slater, on the other hand, was aware that, at long ranges, the generalized virial theorem implied the incipient binding interactions to be due to a kinetic energy lowering, in disagreement with his interpretation.” To suggest that Slater was unaware of any and all physical implications of his molecular virial theorem is inexcusable. Slater gives diagrams for and a full account of the variations in E, T and V in his original 1933 paper and later gave a detailed discussion for H
2
+ and H
2
in his 1963 book
‘Quantum Theory of Solids’. As Slater was the first to demonstrate, the initial decrease in T is a result of the incipient formation of attractive Feynman forces on the nuclei, and is an effect that is precluded from happening at the equilibrium separation.
A study of the spatial behaviour of the kinetic energy density G( r ) was initiated in 1969,
7
demonstrating that it exhibits dramatically low values over the entire internuclear regions of H
2
+ and H
2
. This behaviour is a consequence of the diminished parallel gradients in ρ( r ) because of the accumulation of density over the internuclear region. Thus the accumulation of electron density in the binding region, an accumulation critical to the lowering of the potential energy V necessary for bonding, causes a simultaneous local decrease in the local kinetic energy (KE) density, one that partially offsets its larger increase in the antibinding regions causing the average value of T to equal –E as required by the virial theorem. The KE density G( r ), by providing a real, spatial representation of the kinetic energy, obviates the need to postulate any subjective device to display imagined, model derived decreases in the KE that supposedly occur upon bonding. The dramatic behaviour is unique to the H atom as a consequence of the cusp condition on G( r ).
8
References
1. Hellmann, H. Zur rolle der kinetischen electronenergie für zwischenatomaren kräfte. Z. Physik
35 , 180 (1933).
2. Slater, J.C. The virial and molecular structure. J. Chem. Phys. 1 , 687 (1933).
3. Hellmann, H. Kvantovaya Khimiya. Moscow (1937).
4. Kutzelnigg, W. The physical mechanism of the chemical bond. Angew. Chem. Int. Ed 12 ,
546-562 (1973).
5. Feynman, R.P. Forces in Molecules. Phys. Rev. 56 , 340-343 (1939).
6. Ruedenberg, K. and Schmidt, M.W. Physical understanding through variational reasoning: electron sharing and covalent bonding . J. Phys. Chem. A 113 , 1954-1968 (2009).
7. Bader, R.F.W. and Preston, H.J.T. The kinetic energy of molecular charge distributions. Int. J.
Quantum Chem. 3 , 327-347 (1969).
8. Bader, R.F.W. and Beddall, P.M. A virial field relationship for molecular charge distributions and a spatial partitioning of molecular properties. J. Chem. Phys. 56 , 3320-3329 (1972).