Guidelines For BCH Animal Experiment
advertisement

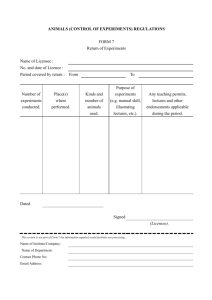
DEPARTMENT OF BIOLOGY AND CHEMISTRY GUIDELINES FOR ANIMAL EXPERIMENT BACKGROUND Teaching and research experiments involving living vertebrate animals can be performed only by staff and students who are licensed under the Animals (Control of Experiments) Ordinance, Cap. 340. A license specifies the type of experiment; type of animal used and place(s) where experiment may be conducted. Any person who contravenes the ordinance shall be guilty of an offence and liable on summary conviction to a fine of one thousand dollars and to imprisonment of six months. Principal Investigators are required to: Follow the “Guidelines on Ethical Review of Research Experiments Involving Animal Subjects” from Research Committee of the University, http://www.cityu.edu.hk/ro, the Guidelines is listed in Appendix I Submit an “Application for ethical review of research experiments involving animal subjects” to Research Committee of the University, the application is listed in Appendix II Apply a license for doing any experiment involving living vertebrate animals, by downloading the form from the website of the Department of Health (as listed in Appendix III): http://www.dh.gov.hk/english/useful/useful_forms/useful_forms_ani.html o Type in all necessary information into the form (Form 1) and the Annex o Obtain a department chop from BCH Chief Technical Officer or Senior Technical Officer o Return the form to Department of Health by mail, fax or email: Office Address: Telephone number: Fax number: E-mail: Special Health Services Department of Health Room 79, 21/F, Wu Chung House 213 Queen’s Road East Wan Chai HONG KONG (Re: Application for License under the Animals (Control of Experiments) Ordinance) 29618975 21277329 ro_al@dh.gov.hk In case of doubt on whether a license is required, advice of the licensing authority should be sought. For teaching experiment, ‘Bloc License’ should be obtained from the Department of Health, by the teaching staff in charge, to cover the group of named students involved in individual experiment(s). -2- Conduct a risk assessment on your experiment(s) (with risk of handling clinical waste and the control measures), risk assessment form can be access from: http://www6.cityu.edu.hk/cdfo/default.aspx?PageID=form&Lang=eng Principal Investigators are requested to send a copy of the ethical review, animal license & risk assessment to the Animal Facility Technical Officer-In-Charge for central filing. TRAINING AND INDUCTION New staff should contact the Animal Facility Technical Officer-In-Charge (TO-InCharge) to take an induction in animal handling techniques which involve viewing a training video, briefing by the Technical Officer-In-Charge followed by a short written test Use appropriate personal protective equipment (e.g. safety goggles, gloves and laboratory coat) at all times PREVENTIVE MEASURES FOR ANIMAL WORKS Bites and scratches o Receive proper training o Wear anti-bite gloves (provided by Department) when handling live animals o Wear surgical gloves, lab coat and safety glasses during dissections and examinations o Immunization against tetanus is strongly recommended o Report as soon as practicable to the laboratory staff, supervisor and Department Head for any bite, scratch or similar injury. Medical advice and subsequent supervision may be needed if an infected animal inflicted the injury Zoonotic Diseases (disease that can be transmitted from animals to humans) o Acquire the animals from reliable sources Links of Animal Centers which supply animal to local universities: HKU http://www.hku.hk/launit/ CUHK http://www.lasec.cuhk.edu.hk/ HKUST http://ihome.ust.hk/~webacf/ o Perform animal manipulations in ventilated biosafety cabinets o No eating, drinking, or apply cosmetics or contact lenses in laboratory o Wear gloves when handling animals or their tissues o Not to accidentally rub the face with contaminated hands or gloves o Wash hands after each animal contact Allergies o Workers may show signs of allergies due to exposures to animals on contact during feeding, cleaning, dosing sacrifice, surgery, and body fluid collection -3- o Symptoms include sneezing and runny nose. More serious reactions include cough, chest tightness, wheezing, or shortness of breath o Workers who report symptoms of work-related asthma should be medically monitored for early intervention o Take following steps to prevent exposures: Perform animal manipulations in ventilated biosafety cabinets Avoid wearing street clothes while working with animals, minimum protection gloves and lab coat should be worn Leaving working clothes at the workplace to avoid potential exposure problems to family members Train workers in recognizing the signs and symptoms of allergic reactions may prevent further asthma development Chemical o Treat anesthetic agents used in laboratory animals as hazardous chemicals with a risk assessment carried out of the chemical agents and the operations involved o Refer to material safety data sheet (MSDS) for the chemical properties of the chemicals used o See http://www6.cityu.edu.hk/cdfo/download/chemicalwastedisposal.pdf for the University policy on the disposal of chemical waste Waste Disposal o Dispose of the bedding and carcasses separately o Seal the bags containing waste with ‘swan neck’ method o Bedding is discarded as general waste while carcasses are stored in designated freezer before they are collected by authorized clinical waste collector USERS’ RESPONSIBILITY Follow the Regulations listed in Appendix IV and V Report any malfunction or facility damage to Animal Facility Technical Officer-InCharge Users are encouraged to work during regular hours (9:00am – 5:30pm) and not to work alone. If you are working outside of these hours or on weekends, you should notify someone of your whereabouts and your expected time of return. No student helpers and undergraduate final-year-project students can work alone in the Animal Facility rooms. They should be accompanied by a postgraduate student or staff at all times Users who need to work after 11:00pm or overnight in laboratory have to inform CDFO at x8888 latest by 5:00pm on the same day. Users have to collect a personal alarm from Ms. Helen Ng, Chief Technical Officer (ext 4080), so as to get emergency help if required Report all incidents and accidents immediately, irrespective of their seriousness to -4- CDFO Security (x8888), Animal Facility Technical Officer-In-Charge, Supervising Academic Staff, and Laboratory Safety Officers STAFF INFORMATION BCH Biological Safety Officer & Animal Facility Staff-In-Charge Dr. Lam Yun Wah Telephone: 34426347 Email: yunwlam@cityu.edu.hk BCH Animal Facility Officer-In-Charge Miss Mok Oi Lam Helen Telephone: 34422030 Email: bhholmok@cityu.edu.hk BCH Safety Officer & Chief Technical Officer Ms. Ng K Y Helen Telephone: 34424080 Email: bhhelen@cityu.edu.hk BCH Senior Technical Officer Mr. Shum K W Eric Telephone: 34424087 Email: bheric@cityu.edu.hk EMERGENCY TELEPHONE NUMBER CDFO Security Hotline Extension: 8888 (24 hours emergency) CDFO Helpdesk Hotline Extension: 8833 (Malfunction of electricity and water supply) -5- Appendix I CITY UNIVERSITY OF HONG KONG Research Committee GUIDELINES ON ETHICAL REVIEW INVOLVING ANIMAL SUBJECTS 1. OF RESEARCH EXPERIMENTS Definitions Principal Investigator (PI) The Principal Investigator is the staff member responsible for the research project. In the case of student research, the supervisor assumes this responsibility. Student Investigator The undergraduate or postgraduate student who will be collecting the research data is referred to as the student investigator. Student Research and Course-based Activities for Student Learning Student research (undergraduate and postgraduate) refers to undergraduate theses, supervised pilot research for an undergraduate or postgraduate thesis, and research that is course-based. Course-based research activities may include: a. students conduct research interviews, administer research tests, or distribute research questionnaires to develop interview of questionnaire design skills b. "mini" research projects where students pose research questions, gather data from human participants, and analyze the data for presentation c. other activities that would be considered research within the disciplinary traditions in which the course is being taught. 2. Licence 2.1 The Animals (Control of Experiments) Ordinance, Cap. 340, of the HKSAR (referred to below as the Ordinance) provides for the control of experiments on living vertebrate animals. No person except a licensee shall perform any experiment involving living vertebrate animals, where a licence is applicable and required under the Ordinance. For this purpose, Section 2 of the -6- Ordinance stipulates that “experiment means any experiment performed on an animal and calculated to give pain”. 2.2 2.3 A licence specifies the place(s) where the experiment may be conducted, its purpose, duration and conditions where appropriate. In addition, special endorsements and permit are required for performing experiments (i) for the purpose of attaining manual skill (Section 4 of the Ordinance); (ii) for the purpose of illustrating lectures (Section 5); (iii) without administering any anaesthetic for killing the animal (Sections 6 & 10). PIs are requested to refer to Cap340 of the Ordinance available on the Government website at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/AA6657F81C8EF4 98482575EE006DEC7E?OpenDocument for other details. In case of doubt on whether a licence is required, advice of the licensing Authority should be sought. Contact details of the Department of Health are as follows: Address: Tel: Fax: Email: Special Health Services Department of Health, Room 79, 21/F, Wu Chung House 213 Queen’s Road East Wan Chai Hong Kong (Re: Application for Licence under the Animals (Control of Experiments) Ordinance) 2961 8645 / 2961 8978 2127 7329 ro_al@dh.gov.hk Application forms for licences can be downloaded from the website of the Department of Health: http://www.dh.gov.hk/english/useful/useful_forms/useful_forms_ani.html. 3. Conduct of Experiments (i) Experiments shall be conducted by appropriately qualified or trained personnel in such a way as to avoid any unnecessary suffering or injury to the animal. -7- 4. (ii) The scientist in charge of the experiment shall allow it to be terminated if its continuation may result in unnecessary suffering or injury to the animal, and shall ensure that authority to do so is delegated at times when he or she is unavailable. (iii) If the experiment or procedure is likely to cause pain and discomfort, the animals shall first be rendered incapable of suffering pain, by anaesthetization, and be maintained in that condition until the experiment or procedure is ended. The only exception to this guideline is where the anaesthetization would defeat the purpose of the experiment and data cannot be obtained by any other procedure. Such experiments or procedures shall be carefully supervised by the Principal Investigator (and must be covered by a special endorsement to a licence - see (a) above). (iv) Post-experiment care of animals shall be such as to minimize discomfort and the consequences of any disability resulting from the experiment. (v) If it is necessary to kill an experimental animal, the animal shall be killed in a humane manner; ie in such a way as to ensure immediate death. No animal shall be discarded until after it is dead. Government Legislation and Codes of Practice 4. 1 Government Legislation Animals (Control of Experiments) Ordinance (Cap. 340) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/AA6657F81C8EF4 98482575EE006DEC7E?OpenDocument Prevention of Cruelty to Animals Ordinance (Cap. 169) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/D2F86B91D0459F CF482575EE0049049A?OpenDocument 4.2 Other related Government Legislation: Dangerous Drugs Ordinance (Cap. 134) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/3E899AC9660B2A CE482575EE00430767?OpenDocument Antibiotics Ordinance (Cap. 137) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/8CA8AB37ACC51 404482575EE00437AB8?OpenDocument -8- Pharmacy and Poisons Ordinance (Cap. 138) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/0F66D4839C78DB DA482575EE00438DD5?OpenDocument Public Health (Animals and Birds) Ordinance (Cap. 139) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/194DC4B24628B1 7C482575EE0043DAB8?OpenDocument Animals and Plants (Protection of Endangered Species) Ordinance, Cap. 187 at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/1460629FC781762 D482575EE004ACBFC?OpenDocument Radiation Ordinance (Cap. 303) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/0209834806B62668 482575EE005B5796?OpenDocument Occupational Safety and Health Ordinance (Cap. 509) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/9198BE222266C42 1482575EF0012128E?OpenDocument For more information, please refer to http://www.legislation.gov.hk 4.3 Code of Practice Code of Practice for Care and Use of Animals for Experimental Purposes (2004) (by Agriculture, Fisheries & Conservation Department) at http://11718-78173.sunnyvisiondatacenter.com/english/quarantine/qua_awc/qua_awc_aw/q ua_am_aw_code/qua_am_aw_code.html Ethical-Animal (guideline) (October 2013) -9- Appendix II CITY UNIVERSITY OF HONG KONG Research Committee APPLICATION FOR ETHICAL REVIEW OF RESEARCH EXPERIMENTS INVOLVING ANIMAL SUBJECTS IMPORTANT NOTES: Please read carefully the following guidelines, government legislation and code of practice BEFORE completing this form. Such references are also available in RO web at http://www.cityu.edu.hk/ro/studentlan/dlAnimal.htm. All sections of the application form must be completed. Please enter “Not applicable” if a section is not relevant. 1) Guidelines on Ethical Review of Experiments Involving Animal Subjects as attached 2) Government Legislation i. Animals (Control of Experiments) Ordinance (Cap. 340) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/AA6657F81C 8EF498482575EE006DEC7E?OpenDocument ii. Prevention of Cruelty to Animals Ordinance (Cap. 169) at http://www.legislation.gov.hk/blis_pdf.nsf/CurAllEngDoc/D2F86B91D0 459FCF482575EE0049049A?OpenDocument iii. Other related government legislation, please refer to Section 4.2 of the attached Guideline on Ethical Review of Research Involving Animals Subjects for information. 3) Code of Practice for Care and Use of Animals for Experimental Purposes (2004) at http://117-18-78173.sunnyvisiondatacenter.com/english/quarantine/qua_awc/qua_awc_aw/qu a_am_aw_code/qua_am_aw_code.html This form must be typewritten. - 10 - Part A: Basic Information 1) Title of Research Project 2) Principal Investigator* / staff in charge of the research project: Name Dept/Unit Post Tel. No. Email Student Investigator/Department (applicable to student project only) Name * Dept/Unit Programme Email In the case of student research, the supervisor assumes this responsibility. 3) Type of Project Grant Type, if any (for staff research) Other (please specify) Funding Agency (if applicable) Thesis Research PhD MPhil Bachelor’s Degree - 11 - Part B: Proposal/Project Details and Methodology (Please complete all sections) 1) Names of Staff/Students Involved in Experiments (a) Name Dept/Unit Post Tel. No. Email (b) Brief description of experience (including types/duration of training attended) of the persons names in Part B 1(a) with regard to animal experimentation: 2) Similarity to previously approved protocol (a) whether the substantive content of this protocol is similar to that of a previously approved protocol (please tick): Yes, please state the number of previous projects that use similar protocols: _____________________________ No (b) If yes, please provide details of the latest similar project : (i) Project title: (ii) Reference no: (iii) Date of Approval: (iv) Capacity : PI / Co-I / Co-PI / Others (please specify) : ______________________ (Please attach animal ethics approval document if available) - 12 - 3) Type of experiment for the currently submitted protocol (please tick) (a) Acute or Chronic Experiment (please tick one) Acute (involving euthanasia of animals only or procedures to be performed on animals under anesthesia followed by euthanasia or procedures to be performed on conscious animals followed by euthanasia; all procedures to be completed within 24 hours) Chronic (involving long-terms [i.e. more than 24 hours] holding animals; procedures to be performed on conscious animals with or without anaesthesia) (b) Non-invasive or Invasive Experiment (please tick one) Non-invasive Invasive (involving puncture or incision of the skin or insertion of an instrument or instrument or foreign material into the body of animals) 4) Types of Licence/Permit/Endorsement Required (Please refer to Section 2 of the Guidelines on Ethical Review of Research Involving Animal Subjects and Cap. 340 for information. Applicants are reminded that licence/permit/endorsement should be obtained from the Department of Health before the commencement of the experiments. Every licensee is required under this Ordinance to submit an annual return to the government on or before the first day of January each year. Please refer to the website of the Department of Health at http://www.dh.gov.hk/english/useful/useful_forms/useful_forms_ani.html for application details. ) a) Type of Licence/Permit/Endorsement Required (please check the box(es) as appropriate) i) Licence to Conduct Experiment alone ii) Endorsement to Authorize Experiments to Attain Manual Skill iii) Teaching Permit (for illustrating lectures) iv) Endorsement to Authorize Experiments without Anaesthetics or without Destroying the Animal b) Application Status of the Licence/Permit/Endorsement (please check the box as appropriate) i) Valid Licence/Permit/Endorsement Approved ii) Application Submitted and being Considered iii) Application NOT yet Submitted (please state the proposed schedule below) _________________________ - 13 - c) If approval is required by other authorities, please complete this section. i) Please indicate if the experiments on live animals are to be carried out in another institution Yes No If yes, please specify the name of the institution: _______________________ ii) Ethical approval involving animals from the institution has been sought: Yes Approval being sought Note: Principal Investigators have to ensure that approval has already been sought from ethics committee of the host institution before commencement of the experiments involving animals. 5) Explanation of how the project advances scientific knowledge and confirmation that alternatives to experiments on live animals have been considered but are not appropriate and/or a reduction of the number of animals to be used have been considered and why such alternatives/reductions are not possible (please attach separate A-4 size sheets if necessary): 6) Species/Strain and Number of Animals to be Used Species/Strain: No. of animals to be used at CityU: No of animals to be used at Collaborating Institute: (only apply if the experiment(s) is/are to be conducted at Collaborating Institute) - 14 - 7) Proposed Location where Animals are to be Kept 8) Place in Which Experiment(s) will be Carried Out 9) The Likely Duration of the Experiments e.g. number of session per year, duration per session. 10) Outline of procedures, with special reference to procedures likely to cause suffering or injury. Experiments should be conducted in such a way as to avoid any unnecessary suffering and injury to the animals. [Please indicate if the protocol being used in this project is the same as the one stated under Part B 2(b). If not, please highlight the differences.] - 15 - 11) Measures to be taken to minimize suffering/injury, including details of anaesthetist, analgesics, euthanasia etc to be used (please specify the relevant guidelines if any, that will be followed, for example, AVMA Guidelines on Euthanasia. 12) Post-experiment Care of Animals - 16 - Declaration (This section must be completed.) I understand that all experiments involving the use of living vertebrate animals must fulfill all relevant legislation and code of practice in Hong Kong and I have read carefully the following guidelines, government legislation and code of practice relevant to the use of animal subjects in experiments including, but not limited to, the following: (please indicate and declare by checking the following boxes.) Guidelines on Ethical Review of Experiments involving Animal Subjects Animals (Control of Experiments) Ordinance (Cap. 340) Prevention of Cruelty to Animals Ordinance (Cap. 169) Other related Ordinances as stated in the Section 4.2 of the Guidelines on Ethical Review of Research Involving Animal Subjects Code of Practice for Care and Use of Animals for Experimental Purposes I certify that I am familiar with and will comply with all rules and legal requirements and pertinent institutional rules/policies on the use of living animals in experiment(s). I shall undertake to exercise reasonable care to ensure that the proposed experiment(s) is conducted in a manner that is consistent with these standards of ethical practice. I understand that failure to observe the University's published protocol may constitute malpractice and be a ground for disciplinary action by the University. Signature (Principal Investigator/Staff/Supervisor) Date To be Completed by Head of Department I endorse this application on the basis of the information provided and the declaration of the PI/Supervisor. Signature (Head of Department) Date Appendix III ANIMALS (CONTROL OF EXPERIMENTS) REGULATIONS FORM 1 Application Form To : The Director of Health I, of on the grounds hereinafter mentioned, hereby apply for — (a) a Licence under section 7 of the Animals (Control of Experiments) Ordinance (Cap. 340). (b) an endorsement / thereto / *to my existing Licence No. * dated / under section 8 of the said Ordinance. (c) a teaching permit under section 9 of the said Ordinance. (d) an endorsement to / the said Licence / *my existing Licence No. dated / under section 10 of the said Ordinance. Grounds for application. Type of experiment(s). Purpose of experiment(s). Place where experiment(s) may be conducted. Qualifications of Applicant and any posts held. Dated Signed *Delete as appropriate. Annex The section below is not part of Form 1 but information supplied would help avoid unnecessary delay in processing your application. Please put a ‘tick’ to the box against each of the followings which are applicable to your application. 1. □ I have not been granted a licence for the experiment under application before; [please go to (2) and (3a or 3b) only] OR □ I am the holder of a valid licence for the experiment under application; (Reference number of licence: ________________ ) [please go to (2) and (4) only] OR □ I am not the holder of a valid licence for the experiment under application but I have been previously granted a licence for the experiment under application which has now expired. (Reference number of latest licence: _______________) [please go to (2) and (5) only] 2. □ I hereby declare that in accordance with Regulations 4 and 5 of the Animals (Control of Experiments) Regulations, Cap.340A (“the Regulations”), I shall keep up-to-date a book in the form set out as Form 6 in the Schedule to the Regulations and I shall render to the Director of Health on or before the 1st day of January each year a return in the form set out as Form 7 in the Schedule to the Regulations of all experiments* performed by me during the preceding twelve months. 3. (a) □ □ □ □ Application for a licence without an “Endorsement to Enable Performance of Experiments without Anaesthetics or without Destroying the Animal” under section 10 of the said Ordinance – I confirm that throughout the whole of the experiment the animal is under the influence of some anaesthetic of sufficient power to prevent the animal feeling pain; and if the pain is likely to continue after the effect of the anaesthetic has ceased, or if any serious injury has been inflicted on the animal, the animal is killed before it recovers from the influence of the anaesthetic which has been administered; AND I confirm that conditions/ well-being of the animals will be monitored during the experiment; AND I confirm that animals with signs of severe distress or pain will be euthanized before the end of the study; AND I confirm that the following method(s) to be used for sacrificing the animals will not cause unnecessary/ prolonged pain to them: □ cervical dislocation ( □ under anaesthesia OR □ not under anaesthesia ) □ decapitation ( □ under anaesthesia OR □ not under anaesthesia ) □ overdose of anaesthetic □ carbon dioxide asphyxiation □ exsanguinations under anaesthesia □ other(s), please specify:________________________________________ (b) Application for a licence with an “Endorsement to Enable Performance of Experiments without Anaesthetics or without Destroying the Animal” under section 10 of the said Ordinance – □ I confirm that the experiment would necessarily be frustrated by – □ the performance of such experiment under any anaesthetic; AND/OR □ killing the animal on which such experiment is performed before it recovers from the influence of any anaesthetic. Please indicate why – 4. □ 5. □ I confirm that the experimental procedures under the type of experiment(s)* and the purpose of experiment(s)* are exactly the same as that of my existing licence under the reference number quoted above; AND □ I will not conduct any experiment* after the expiry date of my existing licence under the reference number quoted above until a new licence is issued AND I have been keeping a proper Form 6 in accordance with regulation 4 of the Regulations. I confirm that the experimental procedures under the type of experiment(s)* and the purpose of experiment(s)* are exactly the same as that of my previous licence under the reference number quoted above; AND □ I hereby declare that I have not conducted any experiment* after the expiry date of my previous licence under the reference number quoted above AND I have kept a proper Form 6 during the validity period of my previous licence under the reference number quoted above in accordance with regulation 4 of the Regulations. “experiment” means any experiment performed on a living vertebrate animal and calculated to give pain (section 2 of the Animals (Control of Experiments) Ordinance, Cap. 340). Full name**: HK Identity Card/Passport/ Travel Document No.: Email Address: Contact No.: Mobile No.: (Facsimile) Signed (Institute/Company chop)*** (Applicant) ** Full name as appears on HK Identity Card/Passport/Travel Document *** Please obtain an official chop of the Institute/ Company where you are working or studying. Appendix IV Regulations for Personnel Working in Animal Holding Room (at Nam Shan Entrance) 1. Access to the animal facility is only limited to authorized persons. 2. All users should wear laboratory gowns and anti-bite gloves when handling animals. 3. Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storing food for human use inside animal holding facility are forbidden. Persons who wear contact lenses should also wear goggles or face shield when working with animals. 4. Door to animal holding room should be kept closed when experimental animals are present. 5. All animal-holding cages must be closed properly. 6. All procedures should be carefully performed to minimize the generation of aerosols. 7. Work surfaces should be decontaminated after use or after any spill of viable materials. 8. Carcasses and used bedding must be properly wrapped up and kept in the designated freezer until collected by licensed clinical waste collector. 9. Personnel should wash their hands after handling animals. 10. Any injury and accident must be reported to the technical staff in charge. Appendix V Regulations for Personnel Working in Rodent Dissection Area (P1702) 1. No housing of rodent is allowed in Rodent Dissection Area. The room and facilities inside are restricted to rodent-associated experiments only. 2. All users should wear laboratory gowns and anti-bite gloves when handling animals. 3. Laboratory gowns or uniforms worn in the Rodent Dissection Area should not be worn in other areas. 4. Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storing food for human use inside Rodent Dissection Area are forbidden. Persons who wear contact lenses should also wear goggles or face shield when working with animals. 5. Door to Rodent Dissection Area should be kept closed when experimental animals are present. The sign ‘Rodent-associated experiment in progress’ should be posted up when there are animal inside Rodent Dissection Area. 6. Cultures, tissues or specimens of body fluids should be placed in leak-free containers during collection, handling, processing, storage and transportation. 7. Broken glassware must not be handled directly by hand, but must be removed by mechanical means such as brushes and dustpans, tongs or forceps. 8. Used needles, sharps and broken glassware should be disposed into designated sharp boxes. 9. Carcasses, isolated animal tissues and wastes must be properly wrapped up and kept in the designated freezer until collected by licensed clinical waste collector. 10. Work surfaces should be decontaminated after use and after any spill of viable materials. 11. Personnel should wash their hands after handling animals and before leaving the Rodent Dissection Area. 12. Any injury and accident must be reported to the technical staff in charge.