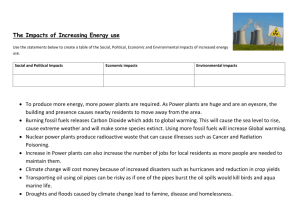
Daviess Taylor Daviess Writing 340 Professor Aubertin 21 February
advertisement

Daviess 1 Taylor Daviess Writing 340 Professor Aubertin 21 February 2014 Harnessing Sunlight A Perfect System Plants seem to have mastered the best and most efficient way to harvest, store and transform energy. If engineers and scientists could find a way to mimic plant processes, solar energy and the overall sustainability of natural resources would be revolutionized. The process through which plants use light and fabricate energy is known as photosynthesis (Figure 1). In photosynthesis, plants convert sunlight or light energy into chemical energy (food) through a biological pathway which facilitates the transmutation of the molecule adenosine triphosphate (ATP) - the bond-breaking energy that the plant utilizes to produce organic compounds such as glucose, water and oxygen. More specifically, plants are able to use sunlight to split hydrogen molecules from water and then store or convert the hydrogen (H2) as a source of energy [1]. This process is known as photocatalysis. Photocatalysis is a reaction that uses light (natural or artificial) to reduces the rate of a chemical reaction without actually bonding with any other reactants or molecules. Basically, it is a chemical reaction that needs a specific amount of light to propel itself forward (activation energy). A catalyst, which is a compound designed to speed up a chemical reaction, will usually be involved to lower the activation energy. This catalyst allows for a reaction to proceed to completion much easier. In plants, this catalyst, a light absorbing dye, is called chlorophyll, which is located in the chloroplasts, Daviess 2 an organelle found at the leaves. Chlorophyll grabs the sunlight and transports it to special ducts where the rest of the photosynthesis process occurs. Artificially Possible? Scientists and engineers have been successful in developing artificial systems that mimic the process of photosynthesis through photocatalyic reactions. However, the devices and research required to perform these reactions require large amounts of money, time and power to operate the lasers and machines. Many universities have laboratories and scientists that continually work on such experiments and reactions, but the scale of obtaining hydrogen is minimal in comparison the energy received from combusting fossil fuels. Therefore, making a complete change to hydrogen as a form of energy would be insufficient with current technology and methods. Despite this fact, there is success in harnessing light and separating hydrogen and oxygen. Scientists are able to do this by utilizing a light source to cause a chemical reaction with water on the surface of a catalyst. Similar to the chlorophyll antennas, scientists and engineers needed to find an effective catalyst that allow artificial photosynthesis to occur and resemble chlorophyll’s mechanism for harnessing light. The most used and researched photocatalyic surface is titanium dioxide (TiO2) because it is nontoxic and fairly cheap. Essentially, hitting light on this surface will excite electrons, causing them to move to a higher energy level. Consequently, this will leave a hole where that electron was initially [2]. These holes then allow for the breaking of water into hydrogen gas (Figure 2). However, there is one main limitation with splitting hydrogen from water: only ultraviolet rays produce the specific wavelength needed to achieve this separation. Daviess 3 Sunlight exhibits a spectrum of light including UV rays. Instead of using the UV rays from the sun, photocatalysis often involves laser stroboscopy to reproduce the light used to drive this artificial photosynthesis. Special lasers have been designed to capture a molecule’s structure and vibration during reactions. In order to study these reactions, the development of lasers with sufficiently fast temporal resolution was essential in studying photocatalysis - because “the initial stages of efficient photochemical reactions invariably occur on the femtosecond (fs) to picosecond (ps) time scale”[3]. The evolution of femtochemistry, how molecules rearrange to form new products, gave rise to such lasers. It provided a way to study a molecule’s structure, vibrations and interactions by shooting pulses of light, with a brief duration of femtoseconds, at a compound or molecule. [4] Essentially, these lasers will shoot a beam of light at a certain wavelength and ultra-fast time to excite an electron to a higher energy level, causing the molecule to vibrate. These vibrations are measured by resonance to observe the dynamics and movements of electrons or atoms within the molecule [5]. When this level of vibration is achieved, it is then probed, or shot with another beam of light, that will split the H2 from water. However, although scientists have methods for carrying out such a task, the exact mechanism through which photosynthesis and the artificial processes split hydrogen is still being researched. Scientists are able to conclude that plants direct light to specific deposits, facilitating a chemical bond, yet the mystery lies in how the plant directs the sunlight. A Race to the Finish Daviess 4 There is a race to discover exactly how plants are able to direct the light energy into the precise deposits or bonds needed to continue the reaction and store the chemical energy converted from the sun. Finding this mechanism is essential to effectively strip and store hydrogen. The main benefit of this breakthrough would be implementation of a more efficient way to produce clean, renewable, and sustainable source of chemical fuel instead of depending on fossil fuel. The U.S. Energy Information Administration reported in 2010 that the United States “consumes more energy than any other nation, derives about eighty-three percent of its energy from fossil fuels: approximately thirty-seven percent from petroleum, twenty-five percent from natural gas and twenty-one percent from coal, with the remaining from nuclear, geothermal, solar, and wind”[6]. It is clear that there are other sources of fuel and energy that deserve attention and resource alteration. And based on this study, it is evident where the U.S. invests its time and money, regarding fuel and energy: fossil fuels. Fossil fuels are substances such as coal, oil and natural gases. We use these materials is so we may obtain the thermal energy, or heat, that is released when we burn the fossil fuels in an exothermic reaction. This heat is then utilized to create and provide our society with fuel and power for necessities such as cars and electricity. Electricity, on a molecular level, is the flow or movement of charged particles via an electrical current. The electrical current is produced by the heat stimulation of electrons in a “hot-potato” sort of way. When the electrons move and pass their excited charge along, an electrical current is created and allows for the possibility of electricity and power. In order to produce electricity and power on an extremely large scale, power plants were designed and allowed for mass production and burning, even though the consequences are extreme. Despite the benefits of being able to light buildings Daviess 5 and highways, power kitchen appliances, and fuel our cars, a change of paradigm is needed to become more self-sufficient, without environmental damage and causing health problems for ourselves. Although this change is necessary, the efficiency that producing and relying solely on solar energy and photovoltaics is generally unknown. Although, we know this form of energy is unquestionably a greater benefit to the environment and overall human health, we do not have as much information on the large-scale production of this form as we do with fossil fuels. We are aware of the negative effects of burning fossil fuels but the power required to sustain and derive hydrogen is currently inefficient to supply America, let alone the world that uses electricity. Why Change? Deriving a new source of fuel is necessary because society has become so reliant on fossil fuels without seriously acknowledging the detrimental effects burning fossil fuel causes to the environment and mankind. First of all, the act of burning these substances releases toxic chemicals into the air we breathe should be sufficient to encourage cessation or reduction of this process. Air pollution is only one major problem with the use of fossil fuels; there are quite a few others. For example, combustion of coal releases smoke and thick fog that look visibly wrong to be breathing. The chemicals released from fossil fuels are sulfur compounds or carbon emissions such as carbon monoxide and carbon dioxide, which increase exponentially with the use of fossil fuels and emission (Figure 3). Increasing these substances in our atmosphere is linked to global warming on top of health related issues. Pollution or contamination from fossil fuels is also extremely harmful to the land and ocean. Oil spills are inevitable with undersea oil drilling. Such Daviess 6 spills take years and years to clean up and are so detrimental to oceans and the living creatures in the ocean that many are dying. On shore damage is also acquiring contamination due to natural gas explosions. Natural gas may have seem like the way to go, because it is “natural” and better than relying on coal and oil. Yet the problem with natural gas lies in its harvesting. Often, there are explosions because methane, CH4, is highly flammable. While the effects on the environment may seem indirect, there is a causal link to the burning of fossil fuels and the health of living beings. In Britain, a study was conducted and published in the British Journal Lancet that reported “air pollution in Austria, France, and Switzerland is responsible for more than 40,000 deaths annually” in those countries alone. “About half of the deaths could be traced to pollution from vehicle emissions,…power plants and other sources of combustion”[7]. The combustion of fossil fuels releases tiny particles into the air. People all over the world, not only in the United States, are experiencing detrimental effects due to these toxic particles. Renewable and sustainable energy are key to the future of the world and mankind’s health. Although some fossil fuels, like oil, are lucrative businesses and help stimulate the economy, the effects of stealing from the earth and then abusing the substance by burning it, result in sicknesses and eventually death. The Daily News published a study on March 6, 2002, relating deaths and illnesses to the inhalation of those tiny particles in Los Angeles County. The report stated that “3,500 deaths each year relate to the inhalation of fine particles.” Furthermore, it was estimated that the economy is being stripped because “nearly two million work days [are] lost to sickness” [7]. As it has been shown, the particles in the air affect our respiratory system as well as our overall health. Reducing Daviess 7 the use and combustion of fossil fuels, are essential to sustaining our health, environment, and economy. Using light as a way to generate and retrieve hydrogen is more reliable and efficient in regards to sustenance of the environment and human condition. Conclusion Renewable energy has been a common discussion throughout the years. Lately, funding for research and the development of sustainable energy has increased. More and more, we are seeing electrical cars, zero emission cars, as well as solar powered lights, etc. Slowly, becoming “green” is hitting the mainstream. Although the exposure and progression toward more sustainable energy is underway, there is still much of work ahead. The reliance and fight for fossil fuels are becoming a bigger problem for the environment, the lives of people and tension between countries. Hydrogen as a source of energy, proves effective in plants and when isolated and burned, proves to be a much more environmentally friendly source of energy. Water is much more accessible and replaceable when compared to fossil fuels and much safer when combusted. Finding the exact mechanism for how plants synthesize hydrogen from water will be a huge step toward more efficient and sustainable energy that causes little to no harm to the environment and our health. Daviess 8 Daviess 9 Figure 1: In photosynthesis, light is absorbed through the leaves, converting carbon dioxide and water into oxygen and glucose (chemical energy). Source: http://sciencebitz.com/?page_id=204 Daviess 10 Figure 2: Photocatalyst utilizes solar or light energy in order to convert water into H2 and ½ O2. Light is absorbed and transmitting through the photocatalyst, releasing H2 and is consequentially split from water, releasing O2 as a byproduct as well. Source: http://www.washington.edu/research/energy/researcher/thomas-g-spiro Figure 3: A byproduct of photosynthesis and burning fossil fuels, CO2, is elevating at an alarming rate and consequentially affecting our atmosphere, oceans, and land. Source: http://search.proquest.com.libproxy.usc.edu/textgraphic/207671740/TextPlusGraphics/D B41FE942BBC4B05PQ/2/8?accountid=14749 Daviess 11 Works Cited Daviess 12 [1] Anonymous Books and arts: The shedding of light; photosynthesis. The Economist pp. 116. 2007. Available: http://search.proquest.com/docview/224009343?accountid=14749. [2] S. L. Gillett. Artificial photosynthesis. Analog Science Fiction & Fact pp. 33-41. 2005. Available: http://search.proquest.com/docview/215341683?accountid=14749. [3] Stanford University, "Ultrafast Chemistry Experiments". Retrieved February , 2013 Available: http://www.stanford.edu/group/pulse_institute/AMD_UltrafastChemistry.shtml [4] S. B. J and A. H. Zewail. Freezing atoms in motion: Principles of femtochemistry and demonstration by laser stroboscopy. J. Chem. Educ. 78(6), pp. 737-751. 2001. Available: http://search.proquest.com/docview/211925544?accountid=14749. [5] Backus, E , "Splitting Water with Sunlight". RetrievedFebruary , 2013 Available: http://www.mpip-mainz.mpg.de/179485/PM8-13eng [6] C. J. Castaneda. Natural disasters in the making: Fossil fuels, humanity, and the environment. Magazine of History 25(4), pp. 21-25. 2011. Available: http://search.proquest.com/docview/902189614?accountid=14749. [7] A. L. Huebner. The cost of fossil fuels. The Humanist 63(2), pp. 7-9. 2003. Available: http://search.proquest.com/docview/235279277?accountid=14749. Images: [1] "Primary and Secondary Productivity". RetrievedFebruary , 2013 Available: http://sciencebitz.com/?page_id=204 [2] Spiro, T , "Solar Hydrogen Production Using Molecular Photocatalys". Daviess 13 RetrievedFebruary , 2013 Available: http://www.washington.edu/research/energy/researcher/thomas-g-spiro [3] S. F. Lincoln. Fossil fuels in the 21st century. Ambio 34(8), pp. 621-7. 2005. Available: http://search.proquest.com/docview/207671740?accountid=14749.