Template for Electronic Submission to ACS Journals
advertisement

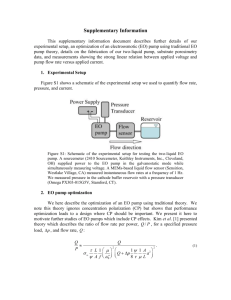
Supplementary Information for Experimental evidence of electroosmotic flow in the region of the electric double layer overlap Hannah Leese and Davide Mattia* Table S1. Summary of Pore Diameters, Thickness and Porosities of the AMM range. Anodization Voltage (V) * 80 80 80 70 70 70 50 50 50 50 40 40 40 30 30 30 25 25 25 20 15 15 10 10 10 24_5 24_5 24_5 24_5 24_5 24_5 error of micrometer ±5μm Pore Diameter, Dp (nm) 80±12 90±14 89±11 80±11 85±10 85±10 67±6 67±6 65±6 52±4 52±4 52±4 50±6 35±6 39±5 36±3 32±3 32±3 30±4 24±4 18±5 18±3 15±3 13±4 15±3 9±3 8±4 8±3 8±3 8±3 8±3 Thickness, L (m)* 85 90 90 55 55 55 45 45 70 60 60 60 80 50 45 40 30 30 30 25 20 20 15 15 15 20 20 20 20 20 20 Porosity, f 0.25 0.28 0.29 0.30 0.25 0.21 0.18 0.18 0.19 0.20 0.18 0.16 0.15 0.11 0.13 0.12 0.13 0.15 0.15 0.12 0.10 0.11 0.09 0.11 0.11 0.09 0.08 0.07 0.07 0.09 0.09 1 Fig. S1: Pore distributions for a) 10 V anodization b) 20 V anodization c) 30 V anodization d) 40 V anodization e) 50 V anodization and f) 80 V anodization. Fig. S2: Linear relationship between anodization voltage and pore diameter, the relation is ~ 1.2 nm V -1. 2 Streaming Potential Measurement The streaming potential measurements were carried out in the electroosmotic rig in Fig.1 to calculate the zeta potential. No voltage was applied across the membrane, but the transmembrane voltage was recorded using a Keithley 179 RMS Digital multimeter when a sequence of pressures was applied to the membrane. The linear relationship of transmembrane voltage and applied pressure enabled the zeta potential of the membrane to be calculated (Eq. 4). An example of the linear relationship between applied pressure and transmembrane voltage has been shown in Fig. S1. The concentration of electrolyte was constant for all measurements for zeta potential calculations and the pH was kept constant; therefore the change in zeta potential was due solely to pore diameter and the increasing interaction between the approaching EDLs. The zeta potential was calculated as follows: d Veff d P S(1) where the conductivity of the liquid. The non-zero intercepts in Fig. S3 (left) arise from asymmetries in the electrodes and have no subsequent effect on evaluating the zeta potentials. The non-zero intercept has also been observed previously (Prakash et al. 2006; Burns and Zydney 2000). The Veff can be calculated using Eq. 3 or by using Eq. 2 with the experimental EOF rates, pore diameter, membrane thickness and zeta potential values. Table S2. Zeta potentials of AAM as a function of pore size. Anodization Voltage (V) Pore Diameter (nm) 15 18±5 20 24±4 30 38±6 40 48±6 50 67±7 Zeta Potential (mV) -16±1 -19±1 -10±1 -17±1 -33±1 Due to the asymmetric nature of the membranes with the smallest pores (<10 nm), a precise measurement of the zeta potential could not be made. While not directly necessary to the calculations performed in this work, a linear extrapolation of the zeta potential values for pores smaller than 10 nm was attempted, based on the results of Fig. 3, where it can be seen that the expression for the total flow rate, QT, shown in Eq. 2 is able to well describe the experimental data for pores above 10 nm. Fig. S3 (left) Streaming potential data for two different AAMs () DP = 67 ±7 nm and (o) DP = 48 ±6 nm at pH 9.2 in 5 mM ionic concentration; (right) Zeta potential as a function of pore diameter; data points to the left of the dashed line (for pore diameters less than 10 nm) are extrapolated. 3 Electroosmotic flow: Effect of applied voltage and electrolyte concentration The electroosmotic flow was found to be independent of pore size for the same effective voltage (Fig. S4 left). On the other hand, EOF increases significantly when varying the buffer concentration (Fig. S4 right, constant V app = 10V). The Debye lengths for 2, 5, 8 and 10 mM were calculated using the Debye Hückel approximation (Eq. 1) and found to be 6, 4.5, 3.5 and 3 nm, respectively. Fig. S4: Electroosmotic pumping performance for three AMMs (● 24 ± 4nm, ○ 52 ± 5 nm, ▼ 67 ± 7nm) as a function of (left) effective voltage, Veff; and (right) of of Na2B4O7 concentration (Vapp = 10 V for all cases). Concentration Polarization and Dukhin number calculations: For a binary electrolyte and AAM the Dukhin number can be expressed as: 𝐷𝑢 = 𝑧1 𝑣1 (𝑠/𝑙) 𝐹𝑐0 (𝑧12 𝑣1 +𝑧22 𝑣2 ) , where the surface charge density, s, was assumed to be spatially uniform and the parameter l is the ratio of total electrolyte volume in the AAM to the pore surface area, c0 is the bulk concentration, v is ionic mobility, z is ionic charge and F Faraday’s constant. The subscripts 1 and 2 represent the counter-ion (Na+) and co-ion (borate) respectively. The surface charge density and ionic mobility were sought from literature (Hernandez et al. 1995; Corti et al. 1980). The Dukhin number is larger than one for all membrane pore sizes examined (Fig. S5) and, as expected, CP effects become significant and cannot be ignored. Fig. S5 Dukhin ratio with pore diameter indicates concentration polarisation propagating within the system. Dukhin numbers > 1 indicate that CP propagates current shocks. References Burns DB, Zydney AL (2000) Buffer effects on the zeta potential of ultrafiltration membranes. Journal of Membrane Science 172 (1–2):39-48 Prakash P, Grissom MD, Rahn CD, Zydney AL (2006) Development of an electroosmotic pump for high performance actuation. J Membrane Sci 286 (1-2):153-160 4