Stevens–Johnson syndrome
advertisement

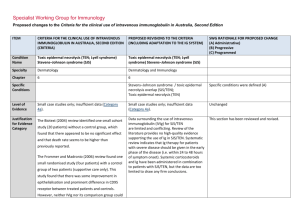
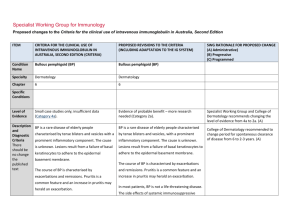
Specialist Working Group for Immunology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED REVISIONS TO THE CRITERIA (INCLUDING ADAPTATION TO THE IG SYSTEM) Condition Name Toxic epidermal necrolysis (TEN; Lyell syndrome) Stevens–Johnson syndrome (SJS) Toxic epidermal necrolysis (TEN; Lyell syndrome) Stevens–Johnson syndrome (SJS) Specialty Dermatology Dermatology and immunology Chapter 6 6 Specific Conditions SWG RATIONALE FOR PROPOSED CHANGE (A) Administrative) (B) Progressive (C) Programmed Stevens–Johnson syndrome / toxic epidermal necrolysis overlap (SJS/TEN); Toxic epidermal necrolysis (TEN) Specific conditions were defined (A) Level of Evidence Small case studies only; insufficient data (Category 4a). Small case studies only; insufficient data (Category 4a). Unchanged Justification for Evidence Category The Biotext (2004) review identified one small cohort Data surrounding the use of intravenous immunoglobulin (IVIg) for SJS/TEN are limited and conflicting. Review of the literature provides no high-quality evidence supporting the use of Ig in SJS/TEN. Systematic review indicates that Ig therapy for patients with severe disease should be given in the early phase of the disease (i.e. within 24 to 48 hours of symptom onset). Systemic corticosteroids and Ig have been administered in combination to patients with SJS/TEN, but the data are too limited to draw any firm conclusions. This section has been reviewed and revised. study (20 patients) without a control group, which found that there appeared to be no significant effect and that death rate seems to be higher than previously reported. The Frommer and Madronio (2006) review found one small randomised study (four patients) with a control group of two patients (supportive care only). This study found that there was some improvement in epithelialisation and prominent difference in CD95 receptor between treated patients and controls. However, neither IVIg nor its comparison group could ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED REVISIONS TO THE CRITERIA (INCLUDING ADAPTATION TO THE IG SYSTEM) SWG RATIONALE FOR PROPOSED CHANGE (A) Administrative) (B) Progressive (C) Programmed SJS and TEN are severe mucocutaneous adverse reactions, most commonly triggered by medications such as sulphonamides, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs) and anti-convulsants. SJS and TEN are characterised by severe bullous reaction with extensive destruction of the epidermis, and morphologically by ongoing apoptotic keratinocyte cell death that results in the separation of the epidermis from the dermis. SJS and TEN are considered a disease continuum and are distinguished chiefly by severity, based upon the percentage of body surface involved with skin detachment. The term SJS describes patients with blistering and skin detachment involving a total body surface area of <10%, SJS/TEN overlap describes patients with 10–30%, and TEN refers to patient with >30% skin detachment. Description and diagnostic criteria have been revised. Yes Which Speciality Unchanged No Which Specialty completely stop the TEN process. Description and Diagnostic Criteria There should be no change the published text TEN is a rare, life-threatening hypersensitivity reaction to certain medications, such as sulphonamides, antibiotics, non-steroidal anti-inflammatory drugs and anti-convulsants. Drug-induced epidermal apoptosis has been proposed as possible pathogenesis. Stevens– Johnson syndrome (SJS) is a less extensive manifestation of the same phenomenon. TEN and SJS are characterised by severe bullous reaction with extensive destruction of the epidermis, and morphologically by ongoing apoptotic keratinocyte cell death that results in the separation of the epidermis from the dermis. The term SJS is now used to describe patients with blistering and skin detachment involving a total body surface area of <10%. SJS/TEN describes patients with 10–30% detachment, and TEN describes patients with >30% skin detachment. Diagnosis is required Diagnosis by a dermatologist; Diagnosis must be verified National Blood Authority Dermatologist pg. 2 ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED REVISIONS TO THE CRITERIA (INCLUDING ADAPTATION TO THE IG SYSTEM) Exclusion Criteria SJS alone SJS alone Indication for use To limit progression of TEN or SJS/TEN when administered in early stages. TEN or SJS/TEN with rapid evolution and >10% body surface area affected. Qualifying Criteria TEN or SJS/TEN overlap with all the following: Erythema and/or erosions affecting >10% body surface area. 1. Diagnosis by a dermatologist; AND 2. Body surface area (erythema and/or erosions) of 10% or more; AND 3. Evidence of rapid evolution. Notes: Urgent skin biopsy should be performed for confirmation but should not delay IVIg therapy if indicated. The Adverse Drug Reactions Advisory Committee should be notified of the inciting National Blood Authority Given that SJS alone is excluded and this condition describes body surface area of <10% involvement, the indication has been modified to exclude SJS alone. Eligibility criteria have been extended to support the early treatment of significant conjunctival lesions and labial blisters rather than waiting for 10% of skin eruptions to develop. (B) Significant mucosal lesions, including conjunctival erosions and labial blisters require early treatment AND Onset of significant skin manifestations (painful red skin with or without blisters and/or any mucosal/conjunctival involvement) has occurred within the last 48 hours. IVIg should be initiated as early as possible, preferably within 24 hours of diagnosis. OR SWG RATIONALE FOR PROPOSED CHANGE (A) Administrative) (B) Progressive (C) Programmed Unchanged IVIg should be initiated as early as possible, preferably within 24 hours of diagnosis. Urgent skin biopsy should be performed for confirmation but should not delay IVIg therapy if indicated. The Adverse Drug Reactions Advisory pg. 3 ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) SWG RATIONALE FOR PROPOSED CHANGE (A) Administrative) (B) Progressive (C) Programmed Committee should be notified of the inciting medication. medication. Review Criteria PROPOSED REVISIONS TO THE CRITERIA (INCLUDING ADAPTATION TO THE IG SYSTEM) N/A There is no review required as this condition requires one-off treatment. Given the one-off dosing - this section will only be completed after treatment and if prescriber enters the data. Outcome measures Clinical assessment one month after immunoglobulin treatment, including: % body surface area still affected diagnostic confirmation by biopsy description of response to Ig treatment. The Adverse Drug Reactions Committee should be notified of the inciting medication. Dose 2 g/kg, preferably as a single dose, or divided over three consecutive days. Dosing above 1 g/kg per day is contraindicated for some IVIg products. Refer to the current product information sheet for further information. The aim should be to use the lowest dose possible that achieves the appropriate clinical outcome for each patient. National Blood Authority Up to 1 g/kg for 3 days within 24 to 48 hours of symptom onset. Up to 2 g/kg as a single dose within 24 to 48 hours of symptom onset. IVIg should be initiated as early as possible, preferably within 24 hours of diagnosis. Skin biopsy should be performed for confirmation, but should not delay IVIg therapy if indicated. Two dosing options are to be offered. From review of the literature, the dosing varies between 2g/Kg as a single dose and 1g/kg for 3 days in the early phase of disease. Systemic corticosteroids and IVIG have been administered in combination to patients with SJS/TEN, but the data are too limited to draw any firm conclusions. No study with set Ig dose and number of days was found. SWG recommendation is to support both dosing of 1g/Kg per day for 3 consecutive days or 2g/Kg as a single dose. This dosing recommendation has been endorsed by the College of Dermatology. (B) pg. 4 ITEM CRITERIA FOR THE CLINICAL USE OF INTRAVENOUS IMMUNOGLOBULIN IN AUSTRALIA, SECOND EDITION (CRITERIA) PROPOSED REVISIONS TO THE CRITERIA (INCLUDING ADAPTATION TO THE IG SYSTEM) SWG RATIONALE FOR PROPOSED CHANGE (A) Administrative) (B) Progressive (C) Programmed Dosing above 1 g/kg per day is contraindicated for some IVIg products. Refer to the current product information sheet for further information. The aim should be to use the lowest dose possible that achieves the appropriate clinical outcome for each patient. POTENTIAL OPERATIONAL IMPACT No operational impacts are anticipated. POTENTIAL IMPACT ON DEMAND Patient Numbers Total treated: 48 2013-14 No impact on demand is anticipated given the very low number of patients treated in any year, despite one dosing option potentially increasing usage by 1g/Kg. POTENTIAL IMPACT ON COST Current cost Anticipated reduction in cost, if any Marginal Marginal = borderline or unchanged from current cost Minor = decrease by $500K - $1.99M from current cost Major = decrease $2M+ from current cost BIBLIOGRAPHY Bachot, N, Revuz, J & Roujeau, JC 2003, ‘Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression’ (comment), Archives of Dermatology, vol. 139, no. 1, pp. 85–6. Biotext 2004, ‘Summary data on conditions and papers’, in A systematic literature review and report on the efficacy of intravenous immunoglobulin therapy and its risks, National Blood Authority pg. 5 commissioned by the National Blood Authority on behalf of all Australian Governments, pp. 242–7. Available from: http://www.nba.gov.au/pubs/pdf/report-lit-rev.pdf. Campione, E, Marulli, GC, Carrozzo, AM, et al 2003, ‘High-dose intravenous immunoglobulin for severe drug reactions: efficacy in toxic epidermal necrolysis’, Acta Dermatovenereologica, vol. 83, no. 6, pp. 430–2. Del Pozzo-Magana, BR, Lazo-Langner, A, Carleton, B, et al. 2011, ‘A systematic review of treatment of drug-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in children’, Journal of Population Therapeutics and Clinical Pharmacology, vol 18, no. 1, pp. e121–e133. Downey, A, Jackson, C, Harun, ,N et al 2012, ‘Toxic epidermal necrolysis: review of pathogenesis and management’ [Review], Journal of the American Academy of Dermatology, vol. 66, no. 6, pp. 995–1003 Frommer, M & Madronio, C 2006, The use of intravenous immunoglobulin in Australia. A report for the National Blood Authority, Part B: systematic literature review, Sydney Health Projects Group, University of Sydney, Sydney, pp. 55–6. Huang, YC, Li, YC, Chen, TJ, 2012, ‘The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis’ [Review], British Journal of Dermatology, vol. 167, no. 2, pp. 424–32. Mockenhaupt, M 2014, ‘Stevens–Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management’ [Review], Seminars in Cutaneous Medicine & Surgery, vol. 33, no. 1, pp. 10–6. Paquet, P, Jacob, E, Damas, P, et al 2005, ‘Analytical quantification of the inflammatory cell infiltrate and CD95R expression during treatment of drug-induced toxic epidermal necrolysis’, Archives of Dermatological Research, vol. 297, no. 6, pp. 266–73. Prins, C, Vittorio, C, Padilla, RS, et al 2003, ‘Effect of high- dose intravenous immunoglobulin therapy in Stevens-Johnson syndrome: a retrospective, multicentre study’, Dermatology, vol. 207, no. 1, pp. 96–9. Trent, J, Halem, M, French, LE, et al 2006, ‘Toxic epidermal necrolysis and intravenous immunoglobulin: a review’, Seminars in Cutaneous Medicine and Surgery, vol. 25, no. 2, pp. 91–3. END OF DOCUMENT National Blood Authority pg. 6