TOPIC 4 : ACID-BASE EQUILIBRIA List three strong acids and three
advertisement
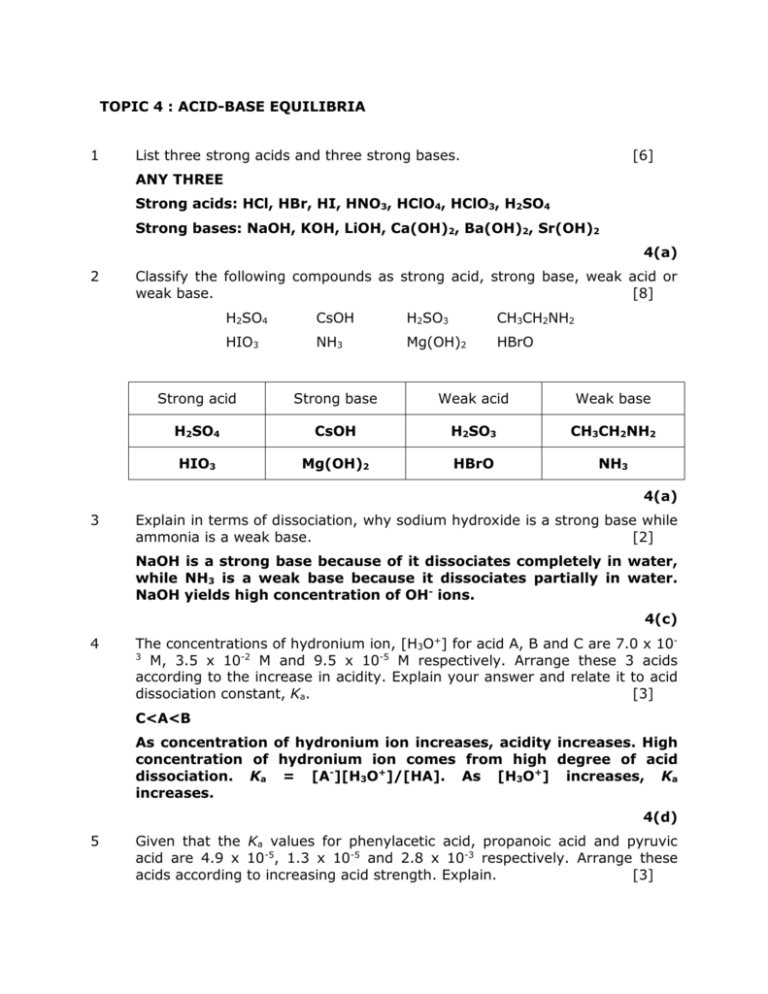
TOPIC 4 : ACID-BASE EQUILIBRIA 1 List three strong acids and three strong bases. [6] ANY THREE Strong acids: HCl, HBr, HI, HNO3, HClO4, HClO3, H2SO4 Strong bases: NaOH, KOH, LiOH, Ca(OH)2, Ba(OH)2, Sr(OH)2 4(a) 2 Classify the following compounds as strong acid, strong base, weak acid or weak base. [8] H2SO4 CsOH H2SO3 CH3CH2NH2 HIO3 NH3 Mg(OH)2 HBrO Strong acid Strong base Weak acid Weak base H2SO4 CsOH H2SO3 CH3CH2NH2 HIO3 Mg(OH)2 HBrO NH3 4(a) 3 Explain in terms of dissociation, why sodium hydroxide is a strong base while ammonia is a weak base. [2] NaOH is a strong base because of it dissociates completely in water, while NH3 is a weak base because it dissociates partially in water. NaOH yields high concentration of OH- ions. 4(c) 4 The concentrations of hydronium ion, [H3O+] for acid A, B and C are 7.0 x 103 M, 3.5 x 10-2 M and 9.5 x 10-5 M respectively. Arrange these 3 acids according to the increase in acidity. Explain your answer and relate it to acid dissociation constant, Ka. [3] C<A<B As concentration of hydronium ion increases, acidity increases. High concentration of hydronium ion comes from high degree of acid dissociation. Ka = [A-][H3O+]/[HA]. As [H3O+] increases, Ka increases. 4(d) 5 Given that the Ka values for phenylacetic acid, propanoic acid and pyruvic acid are 4.9 x 10-5, 1.3 x 10-5 and 2.8 x 10-3 respectively. Arrange these acids according to increasing acid strength. Explain. [3] Propanoic acid<phenylacetic acid<pyruvic acid The higher the Ka value, the higher the degree of dissociation in water. This leads to higher concentration of hydronium ion. Hence, more acidic. 4(d) 6 In each equation, state the conjugate acid-base pairs. [4] (a) NH3 (aq) + HNO3 (aq) ⇌ NH4+ (aq) + NO3- (aq) NH3 + HNO3 ⇌ NH4++ NO3B A CA CB Conjugate acid-base pairs: NH3/NH4+ and HNO3/ NO3- (b) O2- (aq) + H2O (l) ⇌ OH- (aq) + OH- (aq) O2- + H2O ⇌ OH- + OHB A CA CB Conjugate acid-base pairs: O2/OH- and H2O/OH4(b) 7 In each equation, state the conjugate acid-base pairs. [4] (a) NH4+ (aq) + BrO3- (aq) ⇌ NH3 (aq) + HBrO3 (aq) NH4+ + BrO3- ⇌ NH3 + HBrO3 A B CB CA Conjugate acid-base pairs: NH4+/NH3 and BrO3-/ HBrO3 (b) HClO (aq) + H2O (l) ⇌ ClO- (aq) + H3O+ (aq) HClO (aq) + H2O (l) ⇌ ClO- (aq) + H3O+ (aq) A B CB CA Conjugate acid-base pairs: HClO/ClO- and H3O+/H2O 4(b) 8 Write the Ka or Kb expression for the dissociation of the following acid or base. [2] (a) HClO (aq) + H2O (l) ⇌ ClO- (aq) + H3O+ (aq) Ka = [ClO-][ H3O+]/[ HClO] (b) NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq) Kb = [NH4+][OH-]/[NH3] 4(e) 9 Write the equation for dissociation of the following acid or base. State the conjugate acid-base pairs. Write the Ka (or Kb) expressions. [12] (a) HOCN HOCN (aq) + H2O (l) ⇌ OCN- (aq) + H3O+ (aq) Ka = [OCN-][ H3O+]/[ HOCN] Conjugate acid-base pairs: HOCN/OCN- and H3O+/H2O (b) CH3NH2 CH3NH2 (aq) + H2O (l) ⇌ CH3NH3+ (aq) + OH- (aq) Kb = [CH3NH3+][OH-]/[CH3NH2] Conjugate acid-base pairs: CH3NH3+/CH3NH2 and H2O/OH(c) H2S H2S has 2 ionizable protons, hence, it dissociates twice in water. 1st dissociation H2S (aq) + H2O (l) ⇌ HS- (aq) + H3O+ (aq) Ka1 = [HS-][H3O+]/[H2S] Conjugate acid-base pairs: H2S/HS- and H3O+/H2O 2nd dissociation HS- (aq) + H2O (l) ⇌ S2- (aq) + H3O+ (aq) Ka2 = [S2-][H3O+]/[ HS-] Conjugate acid-base pairs: HS-/S2- and H3O+/H2O 4(e) 10 Determine if the following solutions are acidic or basic. Explain. (a) [6] A solution with [OH-] = 9.0 x 10-11 A solution is basic when [OH-] > 1 x 10-7. Thus, this solution is acidic since [OH-] < 1 x 10-7. OR pOH = -log (9.0 x 10-11) pH = 10.04 = 12 – 10.04 = 1.96 Hence, acidic. (b) A solution with [H3O+] = 1.0 x 10-10 A solution is acidic when [H3O+] > 1 x 10-7. Thus, this solution is basic since [H3O+] < 1 x 10-7. OR pH = -log (1.0 x 10-10) = 10 Hence, basic. (c) A solution with pOH of 2.5 A solution with pOH of 2.5 has pH of 11.5. This solution is basic since pH > 7.0 11 Based on the table below, arrange the compounds in order of increasing pH. Show all the calculations involved. [4] pH of X Compound Concentration, M X [H3O+]=1.80 x 10-12 Y [OH-]=2.40 x 10-4 Z [H3O+]=7.20 x 10-6 = -log [H3O+] = -log 1.8 x 10-12 = 11.75 pH of Y = pKw – pOH = 14 – (-log 2.4 x 10-4) = 10.38 pH of Z = -log [H3O+] = -log 7.2 x 10-6= 5.14 Order: Z<Y<X 4(g) 12 Calculate the [OH-] of a solution, given that the [H3O+] is 2.8 x 10-3 M. Determine whether the solution is acidic, basic or neutral. [2] [H3O+]=Kw/[OH-]= 1.0 x 10-14/2.8 x 10-3 M = 3.57 x 10-12 M [OH-] > [H3O+] ; solution is basic 4(f) 13 Calculate the [H3O+] of a solution, given that the [OH-] is 2.8 x 10-3 M. Determine whether the solution is acidic, basic or neutral. [2] [OH-] = Kw/[H3O+] = 1.0 x 10-14/5.7 x 10-11 M = 1.75 x 10-4 M [OH-] > [H3O+] ; solution is basic 4(f) 14 The pH of phenylamine is 8.35. Calculate the [H3O ], [OH ] and pOH of the solution at 25ºC. [3] + pH - =-log [H3O+] [H3O+]=10-pH = 10-8.35 = 4.47 x 10-9 M [OH-] = Kw/[H3O+] = 1.0 x 10-14/4.47 x 10-9 M = 2.24 x 10-6 M pOH = pKw – pH = 14 – 8.35 = 5.65 4(g) 15 The [H3O+] of CH3COOH is 7.2 x 10-6 M. Calculate the [OH-], pH and pOH at 25ºC. [3] [OH-] = Kw/[H3O+] = 1.0 x 10-14/ 7.2 x 10-6 M = 1.39 x 10-9 M pH =-log [H3O+] = 5.14 pOH = pKw – pH = 8.86 4(g) 16 Calculate the pH and pOH 1.0M of HNO3 and 1.0M LiOH of at 25ºC. Determine the similarity between these 2 compounds. [5] Similarity: Both compounds are strong acids (or base), so they dissociate completely in water. (a) 1M HNO3 makes [H3O+] = 1M ; pH = - log [H3O+] = 0 [OH-] = (1 x 10-14)/1 = 1 x 10-14 ; pOH= - log [OH-] = 14 OR (b) pOH = 14 – pH = 14 1M LiOH makes [OH-] = 1M ; pOH = - log [OH-] = 0 [H3O+] = 1 x 10-14/1 = 1 x 10-14 ; pH = - log [H3O+] = 14 OR pH = 14 – pOH = 14 4(g) 17 Calculate the Ka and pKa of 0.010 M HA, given that the [A-] is 1.4 x 10-8 M. [3] [], M HA H2O A- H3O+ I 0.01 - 0 0 C -x - +x +x E 0.01-x - x x From the table, x = [A-] = [H3O+] = 1.4 x 10-8 Ka = [A-][ H3O+]/[HA] = x2/(0.01-x) = (1.4 x 10-8)/(0.01-(1.4 x 10-8)) = 1.96 x 10-14 pKa = -log Ka =13.71 4(h) 18 Calculate the Ka and pKa of 0.80 M HOCN, given that the pH is 1.48. [3] [H3O+]=10-pH = 10-1.48 = 0.033 M [], M HOCN H2O OCN- H3O+ I 0.80 - 0 0 C -x - +x +x E 0.80-x - x x From the table, x = [OCN-] = [H3O+] = 0.033 M Ka = [OCN-][ H3O+]/[HOCN] = x2/(0.80-x) =(0.033)/(0.80 -(0.033)) = 1.42 x 10-3 pKa = -log Ka = 2.85 4(h) 19 Given that the Kb value of 0.5 M of methylamine, CH3NH2 is 4.4 x 10-4. Calculate the concentration of hydroxide ions, OH-. [2] [], M CH3NH2 H2O CH3NH3+ OH- I 0.5 - 0 0 C -x - +x +x E 0.5-x - x x Kb = [CH3NH3+][OH-]/[CH3NH2] = x2/(0.5-x) 0.5/Kb >> 400 ; x is neglected, we assume that [CH3NH2]eq ≈ [CH3NH2]i Hence, the Kb value can be simplified to x2/(0.5) x2/(0.5) x = 4.4 x 10-4 =[OH-] = 0.015 M 4(h) 20 1.0 M of CH3COOH has a Kb value of 5.56 x 10-10. Calculate the concentration of hydronium ion, [H3O+] for this solution. [3] (M) CH3COOH H2O CH3COO- H3O+ I 1.0 - 0 0 C -x - +x +x E 1.0-x - x x Ka=x2/1.0-x Ka= Kw/Kb = (1 x 10-14)/5.56 x 10-10 = 1.8 x 10-5 Ka = [CH3COO-][ H3O+]/[ CH3COOH] = x2/1.0-x 1.0/Ka >>400, x is neglected, we assume that [CH3COOH]eq ≈ [CH3COOH]i x2/1.0 x = 1.8 x 10-5 = 4.24 x 10-3 M = [H3O+] 4(h) 21 Predict and explain whether the following salt is acidic, basic or neutral. [4] (a) CH3COONa Basic salt. CH3COO- is basic because it is derived from a weak acid while K+ is neutral because it is derived from a strong base. CH3COO- will hydrolyze to form OH(b) NH4Cl Acidic salt. NH4+ is acidic because it is derived from a weak base while Cl- is neutral because it is derived from a strong acid. NH4+ will hydrolyze to form H3O+ 4(i) 22 Predict whether lithium bromide, LiBr is an acidic, basic or neutral salt. Explain. [2] Neutral salt. Both Li+ and Br- ions are neutral because they are derived from strong base and strong acid respectively. 4(i) 23 For each of the following, write the relevant acid-base equilibrium equation and predict if pH increases or decreases with each change. Explain your prediction. [4] (a) LiCN is added to HCN (b) NH4Br is added to NH3 (a) HCN (aq) + H2O (l) ⇌ CN- (aq) + H3O+ (aq) Addition of CN- will shift equilibrium to the left (reactant side) and produce more HCN which leads to lesser H3O+. Thus, pH will increase as acidity is reduced. (b) NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq) Addition of NH4+ will shift equilibrium to the left (reactant side) and produce more NH3 which leads to lesser OH-. Thus, pH will decrease as basicity is reduced. 4(j) 24 For each of the following, predict if pH increases or decreases with each change. Explain your prediction. [4] (a) CsOH is added to NaOH (b) KCl is added to HCl (a) Both CsOH and NaOH will dissociate completely in water. Thus, addition of CsOH only adds more OH-, increasing the pH of solution. (b) KCl is a neutral salt. Thus, the addition of KCl has no effect whatsoever on the pH of solution. 4(j) 25 Define buffer. [2] A solution (mixture) of weak acid and its conjugate base or weak base and its conjugate acid that can resist pH changes when a small amount of strong acid or base is added into it 4(k) 26 A 1.0 L buffer solution is made up of 0.80 M HClO2 and 0.40 M NaClO2. [Ka = 1.1 x 10-2] [6] (a) Calculate the initial pH of the buffer solution (b) Calculate the pH if 0.010 mol of KOH is added into the buffer solution (c) Calculate the pH if 0.010 mol of HCl is added into the buffer solution [Assume the volume does not change after each addition] (a) pH = pKa + log [A-]/[HA] = pKa + log [ClO2-]/[HClO2] = -log(1.1 x 10-2) + log (0.4)/(0.8) = 1.66 (b) 0.010 mol of OH- will react with 0.010 mol HClO2 to form 0.010 mol ClO2pH = -log(1.1 x 10-2) + log (0.40+0.01)/(0.80-0.01) = 1.67 (c) 0.010 mol of H3O+ will react with 0.01 mol ClO2- to form 0.01 mol HClO2 pH = -log(1.1 x 10-2) + log (0.40-0.01)/(0.80+0.01) = 1.64 4(m) 27 Calculate the initial pH of a 1.0 L buffer solution that is made up of 0.10 M NH3 and 0.50 M NH4Cl. [Kb = 1.75 x 10-5] [3] Ka =(1 x 10-14)/(1.75 x 10-5) = 5.71 x 10-10 pH = pKa + log [A-]/[HA] = - log (5.71 x 10-10) + log (0.10/0.50) = 8.54 4(m) 28 30 mL of 0.20M propanoic acid, CH3CH2COOH is titrated with 0.10M NaOH. (Ka of CH3CH2COOH = 1.3 x 10-5) (a) Predict whether the pH at equivalence point will be acidic, basic or neutral. (b) Calculate the initial pH (c) Calculate the pH after 10 mL of NaOH is added (d) Calculate the pH after 60 mL of NaOH is added (e) Calculate the pH after 70 mL of NaOH is added (f) Identify a suitable indicator that can be used in this titration (g) Sketch the titration curve (a) Basic. Due to the presence of CH3CH2COO- ion which will hydrolyze to form OH-. (b) [], M CH3CH2COOH H2O CH3CH2COO- H3O+ I 0.20 - 0 0 C -x - +x +x F 0.20 - x - x X 0.20/Ka >>>400. Neglect x, assume [CH3CH2COOH]initial ≈ [CH3CH2COOH]eq Hence, Ka= x2/0.20 x2/0.20 = 1.3 x 10-5 x = 1.612 x 10-3 M = [H3O+] pH = -log [H3O+] = 2.79 (c) n CH3CH2COOH initial = (0.20)(0.03) = 6 x 10-3 mol n OH- added = (0.10)(0.01) = 1 x 10-3 mol n, mol CH3CH2COOH OH- CH3CH2COO- H2O I 6 x 10-3 1 x 10-3 0 0 C -1 x 10-3 -1 x 10-3 +1 x 10-3 0 F 5 x 10-3 0 1 x 10-3 0 pH = pKa + log [CH3CH2COO-]/[CH3CH2COOH] = -log (1.3 x 10-5) + log (1 x 10-3/0.04 L)/(5 x 10-3/0.04 L) = 4.19 (d) n CH3CH2COOH initial = 6 x 10-3 mol n OH- added = 6 x 10-3 mol This is at the equivalence point whereby 6 x 10-3 mol of OH- reacts with 6 x 10-3 mol of CH3CH2COOH to form 6 x 10-3 mol of CH3CH2COO-. CH3CH2COO- will hydrolyze in water as such: n, mol I CH3CH2COO6 x 10-3 H2O CH3CH2COOH OH- - 0 0 C -x - +x +x F 6 x 10-3 - x - x x Kb = Kw/Ka = 1.08 x 10-9 [Initial]/Kb >>> 400. We neglect x and assume [CH3CH2COO-]initial ≈ [CH3CH2COO-]eq [CH3CH2COO-] = 6 x 10-3 mol / 0.09 L = 0.067 M x2/0.067 x = 1.08 x 10-9 = Kb = 8.51 x 10-6 M = [OH-] pOH = -log OH- = 5.07 pH = pKw – pOH = 8.93 (e) This is beyond the equivalence point whereby an excess of 0.010 L x 0.10M = 1 x 10-3 mol OH- is present. [OH-] = (1 x 10-3 mol)/(0.030+0.070)L = 0.01 M pH = 14 – pOH = 14 – (- log 0.01) = 12 (f) Phenolphtalein indicator (g) Sketch of pH-volume curve 29 30 mL of 0.15M NH3 is titrated with 0.10 M HBr. [Kb (NH3) = 1.8 x 10-5] (a) Predict whether pH at the equivalence point will be acidic, basic or neutral. (b) Determine a suitable indicator for this reaction. (c) Calculate the initial pH. (d) Calculate the pH after 10 mL of HBr is added (e) Calculate the pH after 45 mL of HBr is added (f) Calculate the pH after 60 mL of HBr is added (g) Sketch the titration pH curve (a) Acidic pH due to the presence of NH4+ ion which will hydrolyze to form H3O+ (b) Methyl red (c) Initial pH [], M NH3 H2O NH4+ OH- I 0.15 - 0 0 C -x - +x +x F 0.15 - x - x x 0.15 M/Kb >> 400, we can neglect x, we assume [NH3]eq≈[NH3] so the Kb expression can be simplified into Kb = x2/0.15 ; x = [OH-] = 1.64 x 10-3 M ; pH = 14 - pOH = 14 – (-log 1.64 x 10-3) = 11.21 (d) 10 mL HBr n NH3 initial = 4.5 x 10-3 mol n HBr or H3O+ added = 1 x 10-3 mol n, mol NH3 H3O+ I 0.030Lx0.15M 0.010Lx0.10M = 4.5 x 10-3 = 1 x 10-3 NH4+ H2O 0 - C - 1 x 10-3 - 1 x 10-3 + 1 x 10-3 - F 3.5 x 10-3 0 1 x 10-3 - From the table, you can see that this is prior to equivalence point. Also, base and its conjugate acid are present. pH = pKa + log [A-]/[HA] = - log [(1 x 10-14)/(1.8 x 10-5)] + log [(3.5 x 10-3 mol/0.04L)/(1 x 10-3 mol/0.04L)] = 9.80 (e) 45 mL HBr This is at the equivalence point whereby 4.5 x 10-3 mol of HBr reacts with 4.5 x 10-3 mol of NH3 to form 4.5 x 10-3 mol of NH4Br. NH4Br will hydrolyze in water as such: (mol) NH4+ I 4.5 x 10-3 H2O NH3 H3O+ - 0 0 C -x - +x +x F 4.5 x 10-3 - x - x x Ka = Kw/Kb = 5.56 x 10-10 = x2/(4.5 x 10-3-x) ≈ x2/(4.5 x 10-3) ; can be simplified since 0.15/Ka >>400 x = [H3O+] = 1.58 x 10-6 pH = - log 1.58 x 10 -6 (f) ; = 5.80 60 mL HBr This is beyond the equivalence point whereby an excess of 0.015 L x 0.10M = 1.5 x 10-3 mol HBr is present. [H3O+] = (1.5 x 10-3 mol)/(0.045+0.060)L = 0.0143 M pH = - log 0.0143 = 1.84 (g) Sketch of pH-volume curve