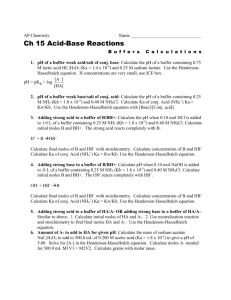
Buffer Problems with answers
advertisement
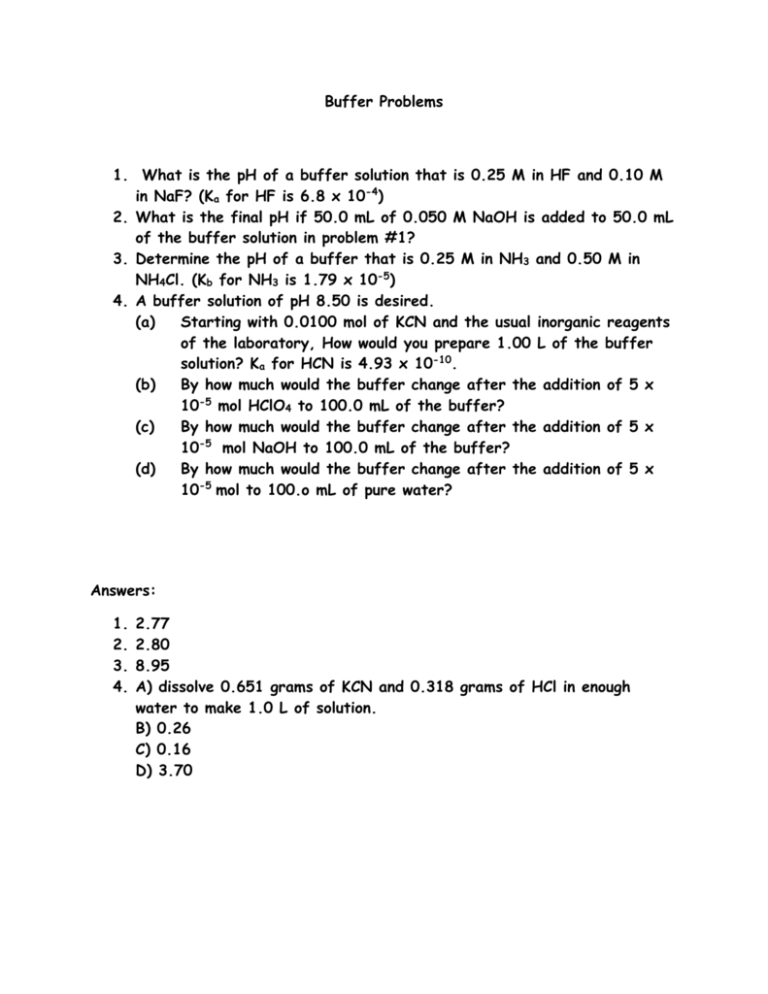
Buffer Problems 1. What is the pH of a buffer solution that is 0.25 M in HF and 0.10 M in NaF? (Ka for HF is 6.8 x 10-4) 2. What is the final pH if 50.0 mL of 0.050 M NaOH is added to 50.0 mL of the buffer solution in problem #1? 3. Determine the pH of a buffer that is 0.25 M in NH3 and 0.50 M in NH4Cl. (Kb for NH3 is 1.79 x 10-5) 4. A buffer solution of pH 8.50 is desired. (a) Starting with 0.0100 mol of KCN and the usual inorganic reagents of the laboratory, How would you prepare 1.00 L of the buffer solution? Ka for HCN is 4.93 x 10-10. (b) By how much would the buffer change after the addition of 5 x 10-5 mol HClO4 to 100.0 mL of the buffer? (c) By how much would the buffer change after the addition of 5 x 10-5 mol NaOH to 100.0 mL of the buffer? (d) By how much would the buffer change after the addition of 5 x 10-5 mol to 100.o mL of pure water? Answers: 1. 2. 3. 4. 2.77 2.80 8.95 A) dissolve 0.651 grams of KCN and 0.318 grams of HCl in enough water to make 1.0 L of solution. B) 0.26 C) 0.16 D) 3.70