Chapter 2 Learning Target 1
advertisement

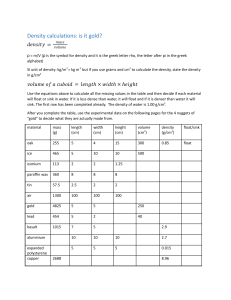
Chapter 2 Learning Target Assessment: LT 1, 2, 3 Name___________________________________ period____________ date___________ LT1:____/20 1. Explain how you can use heat and cold to change the state of matter of a substance. Give a specific example and be sure to talk about what heat and cold would do. A liquid can be changed to a solid by freezing it. A liquid can be turned to a gas by boiling it. 2. Name the 3 common states of matter and explain the movement of particles in each state. The 3 states are liquid, solid and gas. In a solid, the particles only vibrate. In a liquid they slide past each other. In a gas they go everywhere. 3. 4. Matter is everything that has mass and takes up space.. 5. Cross out everything which is not matter: Sound glue energy water air metal gold Thoughts coffee steam fire pickles lead rock snow jello soda paper scissors heat paint love hair ideas diamonds Lt2:____/20 6. Which one of the following is not a physical property: solubility, density, color, odor, flammability, mass? flammability Explain your answer. Flammability is a chemical property because you can’t tell if something will burn just by looking at it. You have to test it. 7. Can you identify the chemical properties of substance just by observing the substance by itself? Why or why not. You cannot observe chemical properties just by looking at the substance because a chemical property is the potential for a substance to change. You have to get the substance to react in order to observe chemical properties. 8. Calculate the density of the following block: Mass = 80g Volume = l x w x h volume = 10cm x 1cm x 2cm V= 20 cm3 Density = mass/volume D = 80g/20cm3 Density = 4g/cm3 9. Explain why the block in #7 would float or sink in the following liquids. Fresh Water: Density = 1.0 g/cm3 The block would sink because it has a greater density than water. Water from the Dead Sea: Density = 124g/cm3 The block would float in the Dead Sea because it has less density than the water there. LT 3____/20 10. Use the following table to identify the unknown substance: substance state color density water liquid clear 1.0 g/ml Sulfuric acid liquid clear 1.8 g/ml Rubbing alcohol liquid clear 0.78 g/ml White vinegar liquid clear 0.96 g/ml unknown liquid clear 1.8 g/ml volume 15 ml 10 ml 23.5 ml 6.5 ml 15 ml The unknown liquid is sulfuric acid. My evidence is that the unknown liquid has the same density as sulfuric acid. Since every substance has a unique density, it must be sulfuric acid. 11. Minerals can be identified by their density. Suppose you had what appeared to be a gold nugget. The density of gold is 19.3 g/cm3. Your piece has a mass of 23 g. Explain how you can find the volume of you nugget which looks like this: Because the nugget has an irregular shape, you would have to use water displacement to calculate the volume. To do this, fill a graduated cylinder to a specific amount. drop the gold nugget into the water. Record the difference in volume. The difference is the volume of the nugget.