submission of post approval documents
advertisement

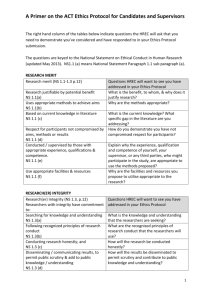
Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC Submissions to Peninsula Health Human Research Ethics Committee (PH HREC) involve an electronic and a hardcopy submission. Please refer to the below for information regarding documents and file formats to be submitted for new applications and for post approval submissions. All electronic submissions to PH HREC are to be emailed to researchethics@phcn.vic.gov.au. For a full list of examples for labelling electronic files for submission, please refer to Appendix 1 Requirements for naming electronic research submissions. SUBMISSION OF POST APPROVAL DOCUMENTS AMENDMENTS Electronic Submission ELECTRONIC FILE FORMAT [HREC Reference Number] Amendment [HREC Reference Number] Amendment Form [Date as dd.mm.yy] (if applicable) [HREC Reference Number] Protocol [Version Number] [Date as dd.mm.yy] [HREC Reference Number] PICF [Version Number] [Date as dd.mm.yy] [HREC Reference Number] Advert (type of ad ie Poster) [Version Number] [Date as dd.mm.yy] [HREC Reference Number] IB [Version Number] [Date as dd.mm.yy] [HREC Reference Number] Questionnaire (Name/Visit) [Version Number] [Date as dd.mm.yy] Repeat for all study related documents When amendment is finalised, either attach all documents to email or send to email and send to researchethics@phcn.vic.gov.au. compressed (zipped) folder, attach to EMAIL TEMPLATE FOR AMENDMENT SUBMISSIONS Subject: [Insert HREC Reference Number] | Electronic Submission of Amendment to PH HREC Email Body (Copy and paste into email): Dear Research Program, I wish to submit an amendment for review by the Peninsula Health Human Research Ethics Committee. HREC Reference: [Insert HREC Reference Number] Research Program Requirements for submission of post approval documents to HREC Page 1 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC Documents for this amendment are attached. A signed hard copy of the amendment form has been forwarded by mail. [If there are any special requirements regarding your application (such as different contact information or upcoming leave which will result in temporary contact via another member of the research team, etc) specify these here] Kind Regards, [Insert Name] [Insert Position on project (Principal Investigator, Associate Investigator)] [Insert Contact Number] Attach to email: Amendment form and supporting documents as required (either individual documents or as a zipped folder). Hard Copy Submission Submit signed amendment form only. Ensure all documents associated with the submission are listed on the form. ADVERSE EVENTS/SERIOUS ADVERSE EVENTS (AE/SAE) Electronic Submission ELECTRONIC FILE FORMAT [HREC Reference Number] AE [HREC Reference Number] AE Report (Internal/External) [Date as dd.mm.yy] (if applicable) [HREC Reference Number] Case Report Form [Date as dd.mm.yy] Repeat for all study related documents When the AE/SAE is finalised attach all documents to email and send to researchethics@phcn.vic.gov.au. EMAIL TEMPLATE FOR ADVERSE EVENT SUBMISSIONS Subject: [Insert HREC Reference Number] | Electronic Submission of AE/SAE to PH HREC Email Body (copy and paste into email): Dear Research Program, I wish to submit an AE/SAE for review by the Peninsula Health Human Research Ethics Committee. Research Program Requirements for submission of post approval documents to HREC Page 2 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC HREC Reference: [Insert HREC Reference Number] All documents relating to this AE/SAE are attached. A signed hard copy of the AE/SAE/SUSAR/USADE Report Form has been forwarded by mail. [If there are any special requirements regarding your application (such as different contact information or upcoming leave which will result in temporary contact via another member of the research team, etc) specify these here] Kind Regards, [Insert Name] [Insert Position on project (Principal Investigator, Associate Investigator)] [Insert Contact Number] Attach to email: AE/SAE/SUSAR/USADE Report Form and supporting documents as required. Hard Copy Submission Submit signed AE/SAE/SUSAR/USADE Report Form only. SUMMARY ADVERSE EVENTS/SERIOUS ADVERSE EVENTS (AE/SAE), SAFETY REPORTS Electronic Submission ELECTRONIC FILE FORMAT [HREC Reference Number] Summary AE [HREC Reference Number] Summary AE Report [Date as dd.mm.yy] [HREC Reference Number] SUSAR Report (Duration ie 6 Monthly, Quarterly) [Date as dd.mm.yy] [HREC Reference Number] Safety Report (Duration ie Annual, Quarterly) [Date as dd.mm.yy] Repeat for any other submission related documents When the Summary AE/SAE, Safety Report is finalised attach all documents to email and send to researchethics@phcn.vic.gov.au. EMAIL TEMPLATE FOR ADVERSE EVENT SUBMISSIONS (COPY AND PASTE INTO EMAIL) Subject: [Insert HREC Reference Number] | Electronic Submission of Summary AE/SAE to PH HREC Email Body (copy and paste into email): Dear Research Program, I wish to submit a Summary AE/SAE for review by the Peninsula Health Human Research Ethics Committee. Research Program Requirements for submission of post approval documents to HREC Page 3 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC HREC Reference: [Insert HREC Reference Number] All documents relating to this Summary AE/SAE are attached. A signed hard copy of the Summary AE/SAE/SUSAR/USADE Report Form has been forwarded by mail. [If there are any special requirements regarding your application (such as different contact information or upcoming leave which will result in temporary contact via another member of the research team, etc) specify these here] Kind Regards, [Insert Name] [Insert Position on project (Principal Investigator, Associate Investigator)] [Insert Contact Number] Attach to email: Summary AE/SAE/SUSAR/USADE Report Form and supporting documents as required. Hard Copy Submission Submit signed Summary AE/SAE/SUSAR/USADE Report Form only. PROGRESS REPORT / FINAL REPORT Electronic Submission ELECTRONIC FILE FORMAT [HREC Reference Number] Progress Report [HREC Reference Number] Progress Report [Date as dd.mm.yy] [HREC Reference Number] Self Audit Assessment Tool [Date as dd.mm.yy] (OR) [HREC Reference Number] Final Report [Date as dd.mm.yy] [HREC Reference Number] Summary of Findings [Date as dd.mm.yy] [HREC Reference Number] Publication (Journal) [Date as dd.mm.yy] Repeat for any other submission related documents When the Progress Report is finalised attach all documents to email and send to researchethics@phcn.vic.gov.au. EMAIL TEMPLATE FOR PROGRESS REPORT/FINAL REPORT SUBMISSIONS Subject: [Insert HREC Reference Number] | Electronic Submission of Progress Report/Final Report to PH HREC Email Body (Copy and paste into email): Research Program Requirements for submission of post approval documents to HREC Page 4 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC Dear Research Program, I wish to submit a Progress Report/Final Report for review by the Peninsula Health Human Research Ethics Committee. HREC Reference: [Insert HREC Reference Number] All documents relating to this Report are attached. A signed hard copy of the Progress Report Form and the Self Audit Assessment Tool has been forwarded by mail. [If there are any special requirements regarding your application (such as different contact information or upcoming leave which will result in temporary contact via another member of the research team, etc) specify these here] Kind Regards, [Insert Name] [Insert Position on project (Principal Investigator, Associate Investigator)] [Insert Contact Number] Attach to email: Progress Report Form and supporting documents as required. Hard Copy Submission Submit signed Progress Report Form and Self Audit Assessment Tool only. PROTOCOL DEVIATION OR VIOLATION Electronic Submission ELECTRONIC FILE FORMAT [HREC Reference Number] Protocol Deviation/Violation [HREC Reference Number] Protocol Deviation or Violation Report Form [Date as dd.mm.yy] (if applicable) [HREC Reference Number] Deviation/Violation Supporting Document [Date as dd.mm.yy] When the submission is finalised attach all documents to email and send to researchethics@phcn.vic.gov.au. EMAIL TEMPLATE FOR PROTOCOL DEVIATIONS OR VIOLATIONS Subject: [Insert HREC Reference Number] | Electronic Submission of Protocol Deviation/Violation to PH HREC Email Body (Copy and paste into email): Research Program Requirements for submission of post approval documents to HREC Page 5 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC Dear Research Program, I wish to submit a Protocol Deviation/Violation for review by the Peninsula Health Human Research Ethics Committee. HREC Reference: [Insert HREC Reference Number] All documents relating to this are attached. A signed hard copy of the Protocol Deviation or Violation Report Form has been forwarded by mail. [If there are any special requirements regarding your application (such as different contact information or upcoming leave which will result in temporary contact via another member of the research team, etc) specify these here] Kind Regards, [Insert Name] [Insert Position on project (Principal Investigator, Associate Investigator)] [Insert Contact Number] Attach to email: Protocol Deviation or Violation Report Form and supporting documents as required. Hard Copy Submission Submit signed protocol Deviation or Violation Report Form only. CORRESPONDENCE Electronic Submission ELECTRONIC FILE FORMAT [HREC Reference Number] Correspondence [HREC Reference Number] Cover Letter (keyword, ie Closure) [Date as dd.mm.yy] (if applicable) [HREC Reference Number] Letter from Sponsor [Date as dd.mm.yy] [HREC Reference Number] Publication (Journal) [Date as dd.mm.yy] Repeat for any other submission related documents When the Correspondence is finalised attach all documents to email and send to researchethics@phcn.vic.gov.au. EMAIL TEMPLATE FOR CORRESPONDENCE Subject: [Insert HREC Reference Number] | Electronic Submission of Correspondence to PH HREC Research Program Requirements for submission of post approval documents to HREC Page 6 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC Email Body (Copy and paste into email): Dear Research Program, I wish to submit correspondence for review by the Peninsula Health Human Research Ethics Committee. HREC Reference: [Insert HREC Reference Number] All documents relating to this is attached. A signed hard copy of the Cover Letter has been forwarded by mail. [If there are any special requirements regarding your application (such as different contact information or upcoming leave which will result in temporary contact via another member of the research team, etc) specify these here] Kind Regards, [Insert Name] [Insert Position on project (Principal Investigator, Associate Investigator)] [Insert Contact Number] Attach to email: Cover Letter and supporting documents as required. Hard Copy Submission Submit signed Cover Letter only. CONTACT Please contact the Research Program if you have any questions regarding submission of applications to the Peninsula Health Human Research Ethics Committee. Research Program Peninsula Health t: (03) 9788 1473 or (03) 9788 1474 e: ResearchEthics@phcn.vic.gov.au w: http://www.peninsulahealth.org.au/research-and-education/human-research-ethics-and-governance/ Research Program Requirements for submission of post approval documents to HREC Page 7 of 9 Version: 11 February 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC APPENDIX 1 – REQUIREMENTS FOR NAMING ELECTRONIC RESEARCH SUBMISSIONS Adapted from Melbourne Health Office for Research requirements for electronic submission: http://www.mh.org.au/www/342/1001127/displayarticle/1001401.html To ensure all electronic files submitted are easily identifiable, please refer to the below tables for the format which must be used to name files relating to your research project. Files submitted that do not follow the format specified may be returned for correct labelling prior to review by the Human Research Ethics Committee. Document Name (File Type) File to be Named As Example HREC Amendment Form (PDF) HREC Reference Amendment Date HREC13PH1 Amendment 01.01.13 AE/SAE/USADE/SUSAR Report Form (PDF) HREC Reference SAE Date HREC13PH1 SAE 01.01.13 Summary AE/SAE/USADE/SUSAR Report Form (PDF) HREC Reference SUSAR Report (Duration) Date or HREC13PH1 SUSAR Report (annual) 01.01.13 HREC13PH1 Safety Report (quarterly) 01.01.13 Protocol Deviation or Violation Report (Word Format) HREC Reference Protocol Deviation Date HREC13PH1 Protocol Deviation 01.01.13 Case Report Form (PDF) HREC Reference CRF Date (Only submit this as when relating to a site related SAE) HREC13PH1 CRF 01.01.13 Progress Report (Word Format) HREC Reference Progress Report (Duration) date or HREC13PH1 Progress Report (Annual) 01.01.13 Final Report (Word Format) HREC Reference Final Report date HREC13PH1 Final Report 01.01.13 Self Audit Assessment Tool (Word Format) HREC Reference Self Audit Date HREC13PH1 Self Audit 01.01.13 HREC Reference Safety Report (Duration) Date Research Program Requirements for submission of post approval documents to HREC Page 8 of 9 Version: 18 March 2013 Research Program | Human Research Ethics Committee REQUIREMENTS FOR SUBMISSION OF POST APPROVAL DOCUMENTS TO HREC Summary of Findings (Word or PDF Format) HREC Reference Summary Findings Date HREC13PH1 Summary Findings 01.01.13 Publication HREC Reference Publication (Journal) Date HREC13PH1 Publication (MJA) 01.01.13 Correspondence Cover Letter (Word Format) HREC Reference Cover Letter Date HREC13PH1 Cover Letter 01.01.13 Letter from PI (Word Format) HREC Reference PI Letter Date HREC13PH1 PI Letter 01.01.13 Letter from Sponsor (Word or PDF Format) HREC Reference Sponsor Letter Date HREC13PH1 Roche Letter 01.01.13 Other Post Approval Attachments List the HREC Reference number followed by document name, version number and date. Where possible, all post approval documents are to be marked with a version number and/or date. Research Program Requirements for submission of post approval documents to HREC Page 9 of 9 Version: 18 March 2013