Unit 2 Review Sheet
advertisement

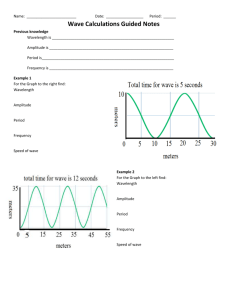
Unit 2 Review Sheet Atomic Theory 1. What is the difference between the law of multiple proportions and definite proportions? 2. What are Dalton’s postulates? 3. Which of Dalton’s postulates are now known to be incorrect? 4. Describe Thomson’s experiment 5. What did Thomson conclude about the electron? (two things) 6. What was incorrect about Thomson’s model? 7. Describe Rutherford’s experiment and what he observed 8. What two things did Rutherford conclude about the atom? 9. What was missing from Rutherford’s model of the atom? 10. Draw Thomson’s model of the atom, Rutherford’s model of the atom, and Bohr’s model of the atom- Label the negative charges and positive charges Atomic Structure 1. A sample of hydrogen is 99% 1H, 0.8% 2H, and 0.2% 3H. What is its average atomic mass? 2. Iodine is 80% 127I, 17% 126I, and 3% 128I. Calculate the average atomic mass of iodine. Atomic # = # of protons. Mass # = Atomic # + neutrons. Protons = electrons when charge is zero. DO NOT LOOK AT THE MASS ON THE PERIODIC TABLE Atomic # Mass # # p+ # e- 1) 17 2) 180 3) 4) 92 40 238 # n0 charge 19 0 71 109 38 46 86 206 5) 6) Symbol Pb4+ 82 34 45 -2 7) 113 8) 21 42 48 49 0 31 P315 9) 10) 83 80 126 108 Ag 47 11) 12) 116 49 +3 13) 128 53 -1 14) 76 188 72 Waves and the Atomic Theory 1. Explain what occurs when energy is absorbed in a metal 2. What is the difference between the ground state and the excited state of the electron? 3. Draw and label the parts of a wave. (including wavelength, amplitude, crest and trough) 4. In each of the following pairs, circle the form of radiation with the LONGER WAVELENGTH: a. red light or blue light b. microwaves or radiowaves c. infrared radiation or red light d. gamma rays or UV radiation 5. Circle the one with the lowest frequency: a. yellow light or green light b. x-rays or gamma rays c. UV radiation or violet light d. AM radio waves or FM radio waves 6. Circle the one with lowest energy a. red light or blue light b. microwaves or radiowaves c. infrared radiation or red light d. gamma rays or UV radiation e. yellow light or green light f. x-rays or gamma rays g. UV radiation or violet light h. AM radio waves or FM radio waves 7. Show ALL equations, work, units in performing the following calculations. (Use your electromagnetic spectrum) C = λν E = hν 8 C = 3.00 x 10 m/s h = 6.626 2 x 10-34 J-s (or J/Hz) 1 eV = 1.602 x 10-19 J 8. What is the wavelength of a wave having a frequency of 3.76 x 1014 s1 ? 9. What is the frequency of a 6.9 x 10-13 m wave? 10. What is the wavelength of a 2.99 Hz wave? 11. What is the wavelength of a 1.28 x 1017 Hz wave? 12. What is the frequency of a 2,600 cm wave? 13. What is the energy of a 7.66 x 1014 Hz wave? 14. 12. What is the frequency of a wave carrying 8.35 x 10-18 J of energy? 15. What is the energy of a 9,330 cm wave? 16. What is the wavelength of a 1.528 x 10-13 J wave? 17. HONORS What is the wavelength of a 1.32 x 10-6 eV wave? Check Notes for Quantum Numbers