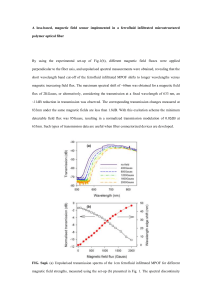
Investigating Ferrofluids - cmaste
advertisement

Investigating Ferrofluids Teacher Background information: Ferrofluids are colloidal fluids of fine ferromagnetic nanoparticles suspended in a carrier fluid (like water or oil). Ferrofluids have a variety of technological uses and applications and are becoming increasingly more common in nanoscience research and industry. The following document includes four investigations of ferrofluids and an STS synthesis activity linking the nanoscience to technologies in society. Connections to Alberta Programs of Study: Science 9: Unit B - Investigate materials, and describe them in terms of their physical and chemical properties. Distinguishing between pure substances, solutions and mechanical mixtures. (Labs 1-4 and STS activity) Physics 30: Unit 3 Concept 1 - Magnetic field theory is a model used to describe magnetic behaviour. (Labs 1-4 and STS activity) Science 30: Unit C Outcome 1 - Students will explain field theory and analyze its applications in technologies. (Labs 1-4 and STS activity) Chemistry 30: Unit B outcome 1 - Students will explain the nature of oxidation-reduction reactions. (Lab / Activity 3 and STS activity) Pedagogical Approach: This lesson includes the following approaches to help build student understanding of ferrofluids and can be used to approach a host of different concepts in the physical sciences. Teachers will: 1) Make connections to student prior knowledge. 2) Help students make macroscopic descriptions from empirical observations. 3) Assist students to render microscopic or particle views of their empirical observations. 4) Help students include symbolic representations to build visual imagery of a concept and better visualize forces and fields that affect materials. 5) Build science literacy with students by identifying new terms and applying definitions to the investigated situations. 6) Make connections with nanoscience in technologies and society. Before labs / activities begin: Start with what students know and what they would like to know. Have them begin the thinking by making drawings of magnetic fields, defining terms they know as well looking up terms they have not heard. Have students make reference to these preliminary thoughts, ideas, questions and drawings at the end of the experiments to reflect on what they have learned. Lab / Activity 1: This activity takes the longest and can be in progress as a kind of demonstration as the students begin their thinking on the topic of ferrofluids. This activity attempts to extract the ferromagnetic oxide coating off of the polymer cassette tapes. Acetone is used to make that extraction as it denatures the binding of the coating allowing it to become free. The tape can then be removed from the acetone and the iron oxide dried. Once dried, vegetable or mineral oil can be added to the iron oxide and tested for magnetic properties. This should produce particles ≥1 μm in size. reference: http://how2dostuff.blogspot.ca/2010/02/how-to-make-ferrofluid-cheap-fast-and.html Lab / Activity 2: The purpose of this short activity is for students to construct a mental image of a magnetic field (sphere of influence). The iron filings help give evidence of that influence. It is intended that in this activity students will draw out their observations on paper and make inferences from them about the nature of a field. Particle size of the iron filings are less 1 mm. Lab / Activity 3: This activity explores the oxidation of iron II into iron III. It helps develop skills in using a buret (needed for titrations) as well as comparing results of reactions (or non reactions) of H2O and 3% H2O2 and iron (II) oxide. The size of the nanoparticles depends on how fine of grade the iron (II) oxide is. *Using a 10% solution of H2O2 is also effective and may decrease the reaction time but it requires additional caution and preparation as the 10% solution is generally prepared by dilution of higher concentrations of H2O2. reference: http://chemeducator.org/sbibs/s0010003/spapers/1030204tm.htm The Chemical Educator, Vol. 10, No. 3, Published on Web 06/01/2005, 10.1333/s00897050908a, © 2005 The Chemical Educator Lab / Activity 4: The idea for this lab is to use MICR toner which contains the ferromagnetic particles and suspend them in a colloidal mixture using oil. The magnetic properties can then be tested for by using a strong magnet. While the oil can weigh down the suspension of particles, look for any peaking or spiking of the fluid. Students will be able to manipulate the fluid using the magnet as well as manipulate its motion when the fluid is placed in an alcohol water mixture. The nanoparticles size is estimated to be larger than 30 nm as the MICR toner is coated with a copolymer that acts as a surfactant. The strength of the magnet and the viscosity of the oil affect the degree of “spiking” of the ferrofluid. Tests using light oils as a carrier fluid (like mineral oil) and strong rare earth magnets appear to yield better “spiking” results. STS Activity: This activity is intended to assist students in making connections with the nanoscience as it applies to various technologies used in society and new nanoscience research. This activity has a list of various applications of ferrofluids. Divide the students into working groups or pairs and have them search out how ferrorfluids are incorporated or used in a specific technology. Have students report their findings to the class. Reporting methods can vary in time and scope: think pair share groups or more formal reports/presentations can be used. Teacher Extension Experiment: There are a few other methods to make ferrofluids teachers can investigate on their own and add to their classroom activities. One such experiment uses magnetite (Fe3O4) 5%, oleic acid - 10% (oleic acid is the surfactant - joins magnetite to carrier fluid), kerosene - 85% (carrier fluid). Making the Fe3O4 Is done by combining ferric chloride (FeCl3) with ferrous chloride (FeCl2), at a controlled rate and in a basic solution produces magnetite (Fe 3O4) nanoparticles (~10nm). 2FeCl3+ FeCl2 + 8NH3+ 4H2O → Fe3O4 + 8NH4Cl The oleic acid surfactant can then be added to the magnetite followed by the addition of the kerosene carrier fluid. http://www.popsci.com/diy/article/2009-09/making-ferrofluids-work-you http://www.youtube.com/watch?v=LlQw9dfexBQ&feature=fvwp Terms and Definitions for these investigations: Ferrofluid Nanoparticle Colloid Surfactant Carrier fluid Magnetic field Magnetic force Disposal of waste ferrofluid: Whatever cannot be saved for recovery or recycling should be managed in an appropriate and approved waste disposal facility. Processing, use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state and local requirements. *High Schools with a laboratory technician should consult them for waste disposal options. Lesson and Lab activities compiled and organized by Ben Oswald - Ross Sheppard High School, Edmonton, AB. Science Teachers consulted for this set of labs and activities: Tracy Onuczko - University of Alberta Kerry-Ann Hyde - St. Joseph High School, Edmonton, AB. ____________________________________________________________________________ __ Ferrofluid Labs and Activities Lab / Activity 1 (or teacher demonstration): Purpose: To extract ferromagnetic oxides and test them for magnetic properties Hypothesis: Design: Ferromagnetic oxides can be removed from the surface of old cassette tapes. The substrate can be collected and tested for magnetic properties. Materials: Acetone - 1 L Beaker - 1 L Hot plate 5-6 tape cassettes (check for brown coating on tape) filter paper funnel and ring stand vegetable oil glass vial or 50 mL beaker Procedure: 1. Disassemble 5-6 tape cassettes 2. place cassette tape in large 1 L beaker 3. in a ventilated room and away from flames, add 500 mL of acetone 4. let it soak for several hours 5. place on a hot plate and heat for 1 hour if needed 6. after cooling, remove the plastic tape from the acetone 7. use filter paper to isolate the iron oxide from the acetone 8. let dry 9. in a small clean dry vial or 50 mL beaker add a small amount of vegetable oil to the dry iron oxide particles (about 3 parts iron oxide to one part oil) 10. Use a strong rare earth magnet to test the ferrofluid for magnetic properties 11. Record your observations Analysis: Evaluation: Lab / Activity 2: Purpose: To map out the magnetic field (a.k.a lines of force) of a strong magnet Design: Iron filings are suspended allowing field lines of a permanent magnet to be detected and mapped. Materials: petri dish clear corn syrup iron fillings electronic balance glass stir rod strong magnet (rare earth) Procedure: 1. Cover the bottom of the Petri dish with corn syrup (approximately 20 mL - just enough to cover the bottom) 2. Using an electronic balance, measure out 2 g of iron fillings 3. Using a glass stir rod, stir the filings into the corn syrup until evenly distributed in the petri dish 4. Bring the magnet close to the bottom of the petri dish 5. Record your observations Evidence: Analysis: Lab / Activity 3: Problem: How does an oxidizing agent affect iron (II) oxide? Design: iron (II) oxide is mixed separately with water and then with hydrogen peroxide. The results are then tested for ferromagnetic properties using a strong magnet. Hypothesis/Prediction: Materials: fine black iron (II) oxide electronic balance 2- 150 mL erlenmeyer flasks 1- buret 3% hydrogen peroxide distilled water Procedure: 1. Using 2 clean 150 mL erlenmeyer flasks measure out and add 0.200 g of black iron oxide (FeO) into each flask. 2. Label each flask 1 and 2 3. Using a clean buret deliver 10 mL of distilled water to one of the flasks 4. Prepare the buret to deliver 3% hydrogen peroxide 6. Deliver 10 mL of hydrogen peroxide to the iron oxide in the second beaker slowly (drop by drop if possible) while swirling 7. Allow both flasks to sit for 30 minutes 8. Using the rare earth magnet test the substance in each flask by bringing the magnet close to the bottom of each flask 9. Record your observations 10. hold the magnet at the bottom to draw as much ferrofluid as possible and decant the peroxide out of the flask. Evidence: Analysis: Evaluation: Lab / Activity 4: Purpose: to create a colloidal mixture of ferrofluid (~ 10 nm nanoparticles) and observe its effects in a magnetic field. Design: Using MICR toner mixed with oil, ferrofluids can reveal the magnetic field of a strong rare earth magnet. Materials: 1 - 50 mL beaker 1 - 100mL beaker 1 - 150 mL beaker MICR toner a light oil like mineral oil (can also use vegetable oil) graduated cylinder stir rod petri dish ferromagnetic bolt or screw (~1/4 inch or more in width) eyedropper rare earth magnet glass vial distilled water isopropyl alcohol Procedure: (Check this . . .add oil to petri dish first then add toner to it, stir??) 1. In a small 50 mL beaker, add 40 mL of MICR Laser jet toner 2. Using a graduated cylinder stir in (approximately) 25 ml of mineral oil using a stir rod 3. Stir until you get an even consistency (more oil can be added if necessary). 4. Put into a 100mL beaker or 150 mL Erlenmeyer flask (a capped vial may also be used). 5. tip the beaker or flask on its side (without spilling the fluid) and bring the rare-earth magnet to the bottom of the flask. 6. Note the shape of the ferrofluid and its influence by the magnetic field. 7. record your observations 8. Place some ferrofluid in a petri dish 9. Place the petri dish on top of the rare earth magnet 10. Note the shape of the ferrofluid. Can a diagram of the magnetic field be drawn? Record your observations. 11. Place an iron screw (standing on end) so that it is magnetised by the magnet. 12. Using an eyedropper place ferrofluid at the bottom of the petri dish onto the screw 13. Note the shape of the ferrofluid 14. Pour a small amount of ferrofluid into a clean 150 mL beaker filled with water 15. Use the rare earth magnet to observe the behavior of the fluid. Record your observations. 16. Using a clean glass vial, fill it 75% with isopropyl alcohol, add a small amount of ferrofluid (~2 mL) and then add the additional 25% distilled water to fill the vial. 17. Tightly cap the vial and use the magnet to influence the ferrofluid. Record your observations. 18. Clean up fluid with soap and warm water Observations: Analysis / Questions: Do you think the mixture is a solid or a liquid? Can liquids be magnetic? What observations did you make when you placed the magnet under the ferrofluid? What are the spikes (if any are noted)? Are the spikes permanent? Is the magnetic effect of a ferrofluid a physical or chemical change? Explain. Where are forces experienced? What are the causes of the forces or interactions? Evaluation or concluding statements: Classroom STS connection activity (Synthesis): The following is an abbreviated list of where ferrorfluids are used in science technology and society. Use the instructions provided by your teacher (as well as the internet) to investigate one of the following. Be prepared to share what you learn according to your teacher’s instructions. algae removal for biofuels, ferrofluid pumps, water purifying systems, electronic devices (high end speaker systems or DVD players), low friction engineering, magnetic drug targeting, MRI enhancement, health research (treating ulcers or cancer research), NASA applications, deformable mirrors, other uses not listed . . .