fwb12507-sup-0001-SupInfo
advertisement

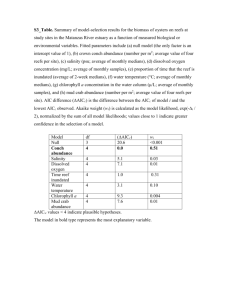
List of materials in supporting information Appendix 1. Description of artificial channel “mesocosm” structure used in experiments and methods used to measure guppy excretion Appendix 2. Details of the experiments combined to generate the dataset used in this paper. Figure S1. A comparison of N excretion (a) and P excretion (b) between the two experiments. Table S1. Characteristics of streams used for guppy-mediated nutrient recycling study. Table S2. Results of model selection procedure for N excretion based on experimental data. Table S3. Results of model selection procedure for N excretion from natural streams. Table S4. Results of model selection procedure for P excretion based on experimental data. Table S5. Results of model selection procedure for P excretion from natural streams. Appendix 1. Description of artificial channel “mesocosm” structure used in experiments and methods used to measure guppy excretion: In our paper we combine data from previously published experiments to make a prediction about how the individual, population and environmental correlates of guppy life history evolution influence nutrient recycling. All of these experiments have used exactly the same artificial experimental channels; here we provide a brief description of the experimental set up, which was described in detail in manuscripts based on these experiments (Bassar et al., 2010; El-Sabaawi et al., In review). Eight flow-through cinder block channels were built adjacent to Ramdeen Stream, a tributary of the Arima River (Trinidad), and were laterally divided into 16 experimental units (mesocosms, 3.0 x 0.5 m). Water was diverted to the mesocosms from a nearby fishless spring via a settling tank, and then delivered to each mesocosm using garden hoses (100 ft long, 3/4 inches wide). Discharge was maintained between 75 L hr-1 and 140 L hr-1 depending on natural spring flow rates, which can vary between seasons and years due to differences in hydrological conditions (e.g. wet season vs. dry season, El-Nino or La-Nina years vs. average years). Water depth was maintained at ~15 cm in each channel. The bottom of each mesocosm was prepared using a commercially available gravel and sand mix that was rinsed to remove silt. An equal mixture of rinsed sand and gravel was evenly spread to a depth of 5 cm at the bottom of each mesocosm. Mesocosms were then conditioned in running water between 7 and 14 days to allow for the build up of biofilm and benthic organic matter. Invertebrates and benthic organic matter were collected using kick nets from the slow-running portions of a nearby tributary of the Arima River. The collection area was equivalent to total mesocosm area. Large larvae of predaceous insects (such as odonates) were removed by hand, and the remaining material was homogenized then distributed equally between all mesocosms. Insects were allowed one week to colonize the mesocosms before each experiment began. In each experiments guppies were collected from natural HP and LP populations, which were then marked using elastomer and introduced into the channels. Each experiment ran for ~28 days, which is enough for the guppies to go through a single reproductive cycle. Similar ecosystem response variables (algal accrual, leaf litter decomposition, invertebrate standing stocks and density) were measured in each experiment using the same methods. Light levels, which were monitored throughout the experiments using HoBo® light loggers and periodic PAR measurements, were controlled by agricultural mesh. In both studies, initial body size distribution and male:female ratios were the same (1:1). The Bassar/density experiments measured the effects of guppy phenotype on ecosystem function in two guppy density treatments. Light levels were kept low (in line with LP sites), but total guppy density was either 24 or 12 guppies per channel, which is representative of guppy densities in LP and HP sites respectively. In the El-Sabaawi/light experiment, density was kept low (12 guppies per channel), but light was varied to reflect light differences in HP and LP sites. The Bassar experiment was executed twice using two different populations (Aripo and Guanapo), but the ElSabaawi experiment only used guppies from the Aripo River. In the paper we only use data from the Aripo experiments. In all experiments and field studies guppy excretion was measured by catching 3-4 guppies from each experimental channel using dipnets, and then placing the guppies in individual ~1 L bags of filtered spring water, which were maintained at ambient water temperature. Ammonium N hr-1 and soluble reactive phosphorous P hr-1 were measured before guppies were introduced in the bag, and and after 20 minutes of incubation. Handling time was estimated prior to the experiments, and excretion trials were constructed to minimize handling stress (Whiles et al., 2009). Ammonium was measured using the fluorometric method (Holmes et al., 1999; Taylor et al., 2007), while phosphorus was measured using the colormetric molybdate method (Parsons, Maita & Lalli, 1984). Each guppy was weighed and measured (standard length). In all experiments guppy excretion was measured on day 27. Literature Cited Bassar R.D., Marshall M.C., Lopez-Sepulcre A., Zandona E., Auer S.K., Travis J., et al. (2010) Local adaptation in Trinidadian guppies alters ecosystem processes. Proceedings of the National Academy of Sciences of the United States of America, 107, 3616-3621. El-Sabaawi R.W., Bassar R.D., Rakowski C., Marshall M.C., Bryan B.L., Thomas S.A., et al. (In review) Intraspecific phenotypic differences in fish affect ecosystem processes as much as bottom-up factors Oikos. Holmes R.M., Aminot A., Kerouel R., Hooker B.A. & Peterson B.J. (1999) A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 56, 18011808. Parsons T.R., Maita Y. & Lalli C.M. (1984) A manual of chemical and biological methods for seawater analysis, Pergamon Press, Oxford Oxfordshire ; New York. Taylor B.W., Keep C.F., Hall R.O., Koch B.J., Tronstad L.M., Flecker A.S., et al. (2007) Improving the fluorometric ammonium method: matrix effects, background fluorescence, and standard additions. Journal of the North American Benthological Society, 26, 167-177. Whiles M.R., Huryn A.D., Taylor B.W. & Reeve J.D. (2009) Influence of handling stress and fasting on estimates of ammonium excretion by tadpoles and fish: recommendations for designing excretion experiments. Limnology and Oceanography: Methods, 7, 1-7. Appendix 2. Details of the experiments combined to generate the dataset used in this paper. Low density means = 12 female guppies per channel, which reflects density in HP sites, and high density = 24 guppies per channel, which reflects density in LP sites. High light = ~68 x 106 and low light = ~16 x 106 lumen ft-1day-1, reflecting values found in HP and LP sites respectively. River is the origin of wild caught guppies used in the experiment. ARP is the Aripo River. Replicates is the number of channels representing each treatment (out of 16 total artificial channels). N is the number of female or juvenile guppies sampled for excretion. Figure S1. A comparison of N excretion (a) and P excretion (b) between the two experiments. The data are from a single treatment, which was identical in both experiments: the low density treatment in Bassar et al. (2010), which was also conducted at low light, and the low light treatment in El-Sabaawi et al. (in review), which was conducted at low density. Light level and guppy density were equal in both experiments. The light experiment (El-Sabaawi et al.) had slightly bigger guppies on average than the density experiment (Bassar et al. 2010), and therefore slightly higher individual excretion rates. There were more pronounced but relatively small differences in P excretion between the experiments in individual excretion rate, as well as the relationship between excretion rate and body size. Phosphorus excretion was slightly higher and more strongly related to body size in guppies from the ElSabaawi/light experiment compared to guppies from the Bassar/density experiment. Log N excretion (ug N per fish per hour) 2.0 A Bassar El-Sabaawi 1.5 Variable Body size Experiment Experimentx body size 1.0 0.5 0.0 -4 -3 -2 -1 F ratio (P value) 45.21 (<0.001) 4.6 (0.04) 0.03 (0.85) 0 Log Body size (wet mass,g) Log P excretion (ug P per fish per hour) 1.5 Bassar El-Sabaawi B 1.0 Variable Body size Experiment Experimentx body size 0.5 0.0 -4 -3 -2 -1 Log Body size (wet mass,g) 0 F ratio (P value) 14.4(<0.001) 46.47(<0.001) 10.93(0.002) Table S1. Characteristics of streams used for guppy-mediated nutrient recycling study. Data are from pools only, which are assumed to be the primary guppy habitat in stream systems. For methods please see El-Sabaawi et al. (2012). Density is the number of guppies per area, measured using depletion curves (CPUE; Catch per Unit Effort). See manuscript for methodological details. Stream Predation Slope Light availability (% open canopy) Arima Aripo Guanapo Marianne HP LP HP LP HP LP HP LP South South South South South South North North 23.4(12.6) 4.4(2.0) 23.6(10.0) 8.3(2.0) 4.9(8.3) 18.1(13.1) 21.1(14.9) 12.3(6.2) Density (m-2) Discharge L.S-1 1.01 3.06 0.27 19.4 2.02 5.02 5.37 N/A 32. 15.8 52.7 41.1 32.6 1323 1478 SRP (g P.L1 ) 82 24.3 34.5 7.7 37.4 44.7 14.6 6.1 Ammonium (g N.L-1) 2.4 0.6 5.8 3.3 3.8 2.8 2.9 1.9 Table S2. Results of model selection procedure for N excretion based on experimental data. Body size is body size lntransformed wet mass (g). D.F. is degrees of freedom. L.L. in the log likelihood ratio. AICc is the corrected Akaike Information Criterion, and ∆AICc is the difference in AIC between model and most parsimonious model. Only models with ∆AICc < 4 are reported. Table S3. Results of model selection procedure for N excretion from natural streams. Body size is body size ln-transformed wet mass (g). D.F. is degrees of freedom. L.L. in the log likelihood ratio. AICc is the corrected Akaike Information Criterion, and ∆AICc is the difference in AIC between model and most parsimonious model. Only models with ∆AICc < 4 are reported Table S4. Results of model selection procedure for P excretion based on experimental data. Body size is body size lntransformed wet mass (g). D.F. is degrees of freedom. L.L. in the log likelihood ratio. AICc is the corrected Akaike Information Criterion, and ∆AICc is the difference in AIC between model and most parsimonious model. Only models with ∆AICc < 4 are reported Table S5. Results of model selection procedure for P excretion from natural streams. Body size is body size ln-transformed wet mass (g). D.F. is degrees of freedom. L.L. in the log likelihood ratio. AICc is the corrected Akaike Information Criterion, and ∆AICc is the difference in AIC between model and most parsimonious model. Only models with ∆AICc < 4 are reported