TOPIC 1 REVIEW
advertisement

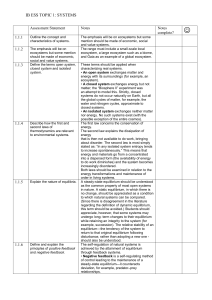
IB ESS Study Guide Topic 1: Systems and models Assessment 1.1.1 Outline the concept and characteristics of systems. 1.1.2 Apply the systems concept on the range of scales. Notes Define system: An assemblage of parts and the relationship between them, which together make a whole. Given a system be able to identify storages (energy or matter), flows (inputs and outputs), and processes (transfer or transformation of energy or matter) Include a small-scale local ecosystem such as a pond a large ecosystem such as a deciduous forest (any biome) Gaia as an example of a global ecosystem. (idea that the whole earth is a living organism) 1.1.3 Define the terms open system, closed system and isolated system. These terms should be applied when characterizing real systems. • An open system exchanges matter and energy with its surroundings (example, an ecosystem). • A closed system exchanges energy but not matter; the “Biosphere II” experiment was an attempt to model this. Strictly, closed systems do not occur naturally on Earth, but all the global cycles of matter, for example, the water and nitrogen cycles, approximate to closed systems. • An isolated system exchanges neither matter nor energy. No such systems exist (with the possible exception of the entire cosmos). 1.1.4 Describe how the first and second laws of thermodynamics are relevant to environmental systems. 1.1.5 Explain the nature of equilibria. The First Law: energy is not created or destroyed (Energy is conserved). 1.1.6 Define and explain the principles of positive feedback and negative feedback. 1.1.7 Describe transfer and transformation processes. 2011 N2 4a 2012 M2 4a 2011 N2 2a 2012 M1 2b The second law explains that as energy is transferred or transformed it becomes less ordered; there is less energy available to do work (the availability of energy to do work is lost as heat) (NOTE: disorder in thermal systems is entropy) Steady-state equilibrium - the common property of most open systems in nature. Static equilibrium, in which there is no change, is a condition to which natural systems can be compared. Some systems may undergo long-term changes to their equilibrium but retain the integrity of the system (example, succession). Stability is the tendency of the system to return to its original equilibrium following disturbance, rather than adopting a new one. The self-regulation of natural systems is achieved by the attainment of equilibrium through feedback systems. • Negative feedback (-) reduces change; a self-regulating method of control leading to the maintenance of a steady-state equilibrium. example, predator–prey relationships. • Positive feedback (+) leads to increasing change in a system—it accelerates deviation example, exponential population growth Transfers flow through a system and involve a change in location (same state) Transformations usually involve a change of state. EXAMPLES Using water as an example, run-off is a transfer process and evaporation is a transformation process. Dead organic matter entering a lake is an example of a transfer process; decomposition of this material is a transformation process. 2013 M1 6b(ii) 2010 M1 1b 2012 M2 4b 2012 N2 3c 2013 M2 1d(iii) 2010 M1 3c 2012 M2 5d 2012 N1 5b 2013 M1 2a(ii) 2012 M2 1c 2011 N1 3b 2011 N2 1b 2011 N1 4a 4b 1.1.8 Distinguish between flows (inputs and outputs) and storages (stock) in relation to systems. Identify flows through systems and describe their direction and magnitude. 1.1.9 Construct and analyze quantitative models involving flows and storages in a system. Storages, yields and outputs should be included in the form of clearly constructed diagrammatic and graphical models. PRACTICE The diagram below shows storages (in percentage of total water) and flows in the global water cycle. The rates of flow are given in 10 15 kg yr –1 . CLOUDS 0.001 % Condensation 400 ATMOSPHERIC WATER VAPOUR 0.001 % Precipitation 100 Evaporation 64 SURFACE WATER 0.001 % ICE 2% GROUND WATER 1 % (a) (i) Precipitation 300 Evaporation 336 Run-off/groundwater flow OCEAN 97 % What is the source of energy which drives the water cycle? ........................ . (1) (ii) In which of the processes given in the diagram does this energy enter the cycle? (1) (b) (i) What percentage of all precipitation falls directly into the oceans? (1) (ii) What percentage of all evaporated water comes from the oceans? (1) (c) (i) Assuming the cycle is in steady state, what mass of water flows into the oceans through run-off and groundwater flow per year? (2) (ii) Explain why this figure might increase in the future, as a result of burning fossil fuels. (3) (d) Name a storage of water in the biosphere that is not shown in the diagram, and explain how water is transferred in and out of this storage. (3) (e) (i) State briefly one way in which one of the other flows in the diagram might change if evaporation rates were to increase. (1) (ii) Describe how two changes in the flows shown on the diagram could lead to a fall in global temperatures and reduce global warming. (4) (iii) Name the type of feedback involved in this reduction in global warming. (1) (f) Identify each of the different processes referred to on the diagram as either transfer or transformation processes. (2) 1.1.10 Evaluate the strengths and limitations of models. A model is a simplified description of the structure or workings of an object, system or concept. Examples: model of climate change an aquarium models a simple ecosystem Strengths of Models allow scientists to simplify and communicate complex systems allow scientists to make predictions inputs can be changed and outcomes examined without having to wait for real events. results can be shown to scientists and the public Limitations of Models might not be totally accurate rely on the expertise of people making them different people may interpret them in different ways model may be based on unreliable or incomplete data different models may show different effects using the same data PRACTICE 1. Which of the following factors would prevent the ecosphere being classified as a closed system? A. The input of solar energy B. The re-radiation to space of heat energy C. The arrival of rocks as meteorites from space D. The unstable state of its equilibrium 2. Leaching of soil nutrients is an example of A. transfer of materials. B. transformation of materials. C. transfer of energy. D. transformation of energy. 3. Which of the following contributes most effectively to self-regulation within a system? A. Rapid transfer of materials B. Inputs of energy being greater than outputs C. Negative feedback mechanisms D. Positive feedback mechanisms E. 4. A lake with a stream flowing into it, but with water lost only by evaporation, is an example of a system which is A. isolated. B. stable and closed. C. unstable and closed. D. open. 2010 M1 1c 2013 N1 1c(iii)