SOP: Plaque Assay - University of Florida
advertisement

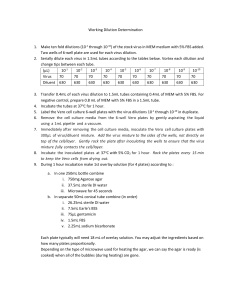
Date Issued: Jan 15, 2014 SOP: Plaque Assay Global Pathogen Laboratory Emerging pathogen Institute University of Florida SOP: Plaque Assay One Health Center of Excellence Emerging pathogen Institute University of Florida I. PURPOSE: This SOP provides detailed information on performing plaque assay which is primarily used to determine the number of infectious viral particles in a sample as plaque-forming units (pfu/mL). the procedure would be used to identify viral titer in pfu/ml units for each of West Nile virus (WNV), dengue virus (DENGV), Chikungunya virus (CHIKV) or Japanese encephalitis virus (JPV). Please read carefully all the below steps prior to processing any of your samples. Consult your supervisor or University of Florida before varying any part of this protocol II. PRECAUTION: This procedure involves working with viral isolates of life threatening pathogens. You must be familiar with University of Florida biological safety regulations. Use the precautions for BSL-2/3 agents. Always wear gloves and a lab coat. Do all work in a biological safety cabinet (BSC). Properly disinfect the work area with 2X CiDecon, 10% bleach or SaniWipes® followed by 70% ethanol to remove any residual cleaning residue. Dispose all materials that contact specimens in the biohazard waste containers and autoclave all infectious materials before final disposal. III. Procedure: The four viruses of interest all grow on the same cell line/s, please refer to the cell culture SOP for preparing 6 well plate with 90% confluent monolayer of VERO cell line. I. Plan to infect cells when they are 90% confluent. II. Remove growth media by gently aspirating from the side of the well with a thin-tipped pipette using a pipet aid. III. Wash the cells with 100ul of media in each well, rock the plate gently to allow maximum coverage of the media to all cells then aspirate one more time. IV. Prepare Virus Stock Dilution: i. Prepare a 10-fold serial dilution of viral-stock with 10 different dilutions(figure1) ii. Inoculate a 300ul of each dilution into each well. iii. Accurately label the cover of the plate with the corresponding well dilution. iv. Incubate at 36 oC and 5% CO2 for an hour, make sure to rock the plate every 20 minutes to avoid cell drying. Figure (1) http://www.virology.ws/2009/07/06/detecting-viruses-the-plaque-assay/ Date Issued: Jan 15, 2014 v. vi. vii. Global Pathogen Laboratory Emerging pathogen Institute University of Florida SOP: Plaque Assay Prepare overlay by mixing Avicel with 2XMEM + 20% FBS at a 1:1 ratio, then vortex vigorously until fully dissolved. Add 1ml of the previous overlay mixture to each well. Incubate at 36 oC with 5% CO2 for 4-24 hours depending on the virus type. (Hint: please mention incubation time per virus) Working dilutions of the stock virus in MEM medium with 5% FBS will be prepared according to the below table. Each dilution will be used in two wells and the average count of the two wells will be used. (µL) 10-1 Virus 70 Diluent 630 V. 10-2 70 630 10-3 70 630 10-4 70 630 10-5 70 630 10-6 70 630 10-7 70 630 10-8 70 630 10-9 70 630 10-10 70 630 Agar Overlay 1. Prepare a sterile solution of 4% agarose in ddH2O by autoclaving at 121 oC for 30 minutes as follows: A. in a 250 mL bottle combine the following: i. 1g Agarose agar ii. 50 mL sterile deionized water (DI water) iii. Microwave for 30-45 seconds, avoid allowing the flask to boil in the microwave, open frequently and rock to allow maximum mix and reduce evaporation time. iv. The agar is completely melt if no observed bubbles are seen in the bottle. B. in a separate 250 mL bottle combine the following (in order) i. 35mL sterile DI water ii. 10mL Earle’s BSS iii. 100ul gentamicin iv. 2mL FBS v. 3mL sodium bicarbonate 2. 3. 4. 5. Warm MEM in a 37◦C water bath. Give the media enough time to be completely warmed up into 37◦C before adding it to your cells. Place the mix into a 37◦C water bath all the time. Gently remove media from the infected monolayer cells using fine pipette. Avoid touching the monolayer with the pipette to prevent cell shearing. (you may use an aspirating pipette attached to a vacuum) Mix the media with the agar under sterile conditions. Figure (1) http://www.virology.ws/2009/07/06/detecting-viruses-the-plaque-assay/ -Ctrl 0 630 SOP: Plaque Assay Date Issued: Jan 15, 2014 6. 7. 8. 9. VI. Global Pathogen Laboratory Emerging pathogen Institute University of Florida Underneath a biosafety cabinet, add 3ml of 1st overylay at the side of each well. Allow the plate to sit in the hood for 15 minutes at room temperature until the agar overlay solidify. Move the plate into a humidified incubator at 36◦C with 5% CO2 upside down. Incubate for 72 hours. Second Overlay: 1- Prepare the 2nd overlay as follow: a) Preparation of Neutral Red Stock Solution: i. 151 mg neutral Red Crystal/powder + 100mL DIH2O ii. Autoclave at 121 oC for 10 min and store at 4 oC iii. Since neutral red dye is photosensitive, always cover the bottles with aluminum foil before storing at 4oC 23456- b) Prepare neutral red solution for 2nd overlay: I. Mix 3mL of neutral red stock solution with 100mL PBS Add 3mL of neutral red solution for 2nd overlay on the side of the wall of each well. (turn off the hood light in this step to avoid stain degradation) Incubate the 6-well plates at 37oC with 5% CO2 for 6hrs. Aspirate the neutral red staining solution without disrupting the agar overlay. Continue to incubate overnight at 37oC. Examine the plates for plaques. a. Plaque Visualization: i. Plaque forming unit (PFU/mL): a virus or group of viruses that cause a plaque. ii. Count the number of plaques formed in each well (remember to look on the plate from the bottom of the wells i.e. inverted) over a light box. iii. Calculate the concentration of the initial viral solution inoculated on the single monolayer to determine PFU/mL i.e. No. of Plaques = pfu/mL = plaques X D X V D = Dilution Factor V= Volume of diluted virus added to the well. Example: if you inoculated 100uL of dilution 10-6 in a well and you counted 44 plaques. The titer from that well is 106 X 44 = 4.4X107 PFU/100uL (the initial inoculum), you may multiply the this number by 10 to obtain PFU/mL to be 4.4X10-8PFU/mL Figure (1) http://www.virology.ws/2009/07/06/detecting-viruses-the-plaque-assay/ Date Issued: Jan 15, 2014 IV. V. SOP: Plaque Assay Global Pathogen Laboratory Emerging pathogen Institute University of Florida If plaques are above 60 consider them too numerous to count TNTC and avoid using this concentration to determine your plaque. To perform your own plaque forming dilution use the following Equation: (Plaques Counted) X (Dilution Used) = (Plaques desired) (Dilution Required) Example: 1 : 10,000 virus dilution produced more than 100 plaques, and the 1 : 100,000 virus dilution produced 30 plaques, the estimated virus dilution needed to produce 50 plaques can be calculated as follows (30) (100,000) = (50) (X) X = 3,000,000 divided by 50 X = 60,000 A 1 : 60,000 dilution of virus should yield about 50 plaques. This dilution, when used as the stock virus to prepare PRNT, is referred to as the “working dilution” (wd). Appendix I: Equipment: Incubator with CO2 gas chamber 37oC Biosafety Cabinet Water Bath Microwave 37oC/56oC Refrigerator 100 – 250 mL bottles and flasks Tissue Culture Plates (Polypropylene) CO2 cylinder Pipet aid Micropipette tips : 0-200uL, 0-1000uL Sterile dilution tubes Vortex Ice Maker machine Appendix II: Reagents: 1. Complete Growth medium for VERO cell-line (Minimal Essential Media) 2. Agarose Gel 3. Neutral Red Figure (1) http://www.virology.ws/2009/07/06/detecting-viruses-the-plaque-assay/ Date Issued: Jan 15, 2014 SOP: Plaque Assay Figure (1) http://www.virology.ws/2009/07/06/detecting-viruses-the-plaque-assay/ Global Pathogen Laboratory Emerging pathogen Institute University of Florida