Kalydeco™ (ivacaftor)
Prior Authorization
(with Quantity Limit) Program
Summary
This program applies to GenRx open, GenRx closed, FlexRx open and FlexRx closed formularies
FDA APPROVED INDICATIONS AND DOSAGE
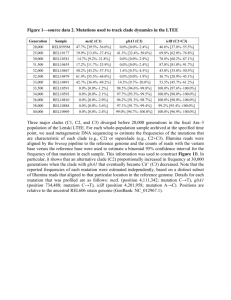
FDA Indication1: For the treatment of cystic fibrosis (CF) in patients age 6 years of age and
older who have G551D mutation in the CFTR gene. If the patient’s genotype is unknown, an
FDA-cleared mutation test should be used to detect the presence of the G551D mutation.
Ivacaftor is ineffective in patients that are homozygous for the F508del mutation in the CFTR
gene.
Dosing:
The recommended dose is 150 mg orally every 12 hours with a fat-containing food. Dose
reductions are recommended for hepatic impairment and when co-administered with moderate
to strong CYP3A4 inhibitors. Co-administration with CYP3A4 inducers (e.g. rifampin, St. John’s
Wort) is not recommended.
CLINICAL RATIONALE2
CF is a life threatening, inherited condition that affects the cells that produce mucus, sweat and
digestive enzymes. CF is caused by defects in the CFTR (cystic fibrosis transmembrane
regulator) gene which encodes for a protein that functions as a chloride channel and regulates
the flow of other ions across the surface of epithelial cells. Mutations in the CFTR gene result in
abnormalities of chloride transport across epithelial cells on mucosal surfaces. The failure results
in chloride and water transport abnormalities which causes viscid secretions in the respiratory
tract, pancreas, gastrointestinal tract, sweat glands, and other exocrine issues. The increased
viscosity makes these secretions hard to clear. Thus far 1,893 CTFR mutations have ben
identified with half of all individuals of northern European descent have the ∆F508 mutation.
Another 25%-30% have one copy of ∆F508 plus another mutation. About 4% of those with CF
(roughly 1,200) people are believed to have the G551D mutation. There is no cure for CF but
antibiotics, mucus-thinning drugs and bronchodilators can ease symptoms and reduce
complications.
Efficacy1
The efficacy of ivacaftor was evaluated in two randomized, double-blind, placebo-controlled
trails in 213 CF patients with the G551D mutation. In both trials patients were randomized to
either 150 mg of ivacaftor twice daily or placebo. The primary efficacy endpoint in both trials
was improvement in lung function as determined by the mean absolute change from baseline
in percent predicated per-dose FEV1 through 24 weeks of treatment. Trial 1 was evaluated in
161 patients 12 years of age and older with baseline FEV 1 between 40-90% predicted [mean
FEV1 64% predicted (range: 32%-98%)]. Trial 2 evaluated 52 patient’s age 6 to 11 years old
with a baseline FEV1 between 40-105% predicted [mean FEV1 84% predicted (range: 44% to
134%)]. The treatment difference in mean absolute change in percent of FEV 1 between
ivacaftor and placebo at week 24 in Trial 1 was 10.6% (P<0.0001) and 12.5% (P<0.0001) in
Trial 2. These changes persisted through week 48. In both studies treatment with ivacaftor
resulted in significant improvement in FEV1.
Safety1
MN_CS_Kalydeco_PA_ProgSum_AR1112.doc
© Copyright 11/2012 All Rights Reserved
Page 1 of 3
The most common adverse events based on pooled data from clinical trials in 213 patients
include headache, upper respiratory tract infection, nasal congestion, nausea, rash, rhinitis,
dizziness, arthralgia and bacteria in sputum. There is potential for ivacaftor to cause elevated
liver enzymes. These should be monitored at baseline and every 3 months. There are no
contraindications to therapy with ivacaftor.
For additional clinical information see the Prime Therapeutics Formulary Monograph.
REFERENCES
1. Kalydeco prescribing information. Vertex Pharmaceuticals, Corporation. January 2012.
2. Kalydeco Monograph. Prime Therapeutics. January 2012.
MN_CS_Kalydeco_PA_ProgSum_AR1112.doc
© Copyright 11/2012 All Rights Reserved
Page 2 of 3
Kalydeco™ (ivacaftor) Prior Authorization (with Quantity Limit)
OBJECTIVE
The intent of the prior authorization (PA) requirement for Kalydeco™ is to encourage
appropriate selection of patients for treatment according to product labeling and/or clinical
studies and/or guidelines and according to dosing recommended in product labeling. Criteria
will limit the approved dose for Kalydeco to at or below the maximum FDA labeled dose.
TARGET DRUGS
Kalydeco™ (ivacaftor)
QUANTITY LIMIT TARGET DRUGS- RECOMMENDED LIMITS
Brand (generic)
GPI
Multisource Code
Kalydeco™ (ivacaftor)
150 mg tablet
45302030000320
M, N, O, or Y
Quantity per
Day Limit
2 tablets
PRIOR AUTHORIZATION CRITERIA FOR APPROVAL
Kalydeco – INITIAL evaluation will be approved when the following are met:
1.
The patient has a diagnosis of cystic fibrosis AND
2.
ALL of the following:
a. The patient has the G551D mutation of CFTR gene as confirmed by genetic
testing AND
b. The patient is not homozygous for the F508del mutation AND
c. The patient is at least 6 years old AND
d. ONE of the following:
i. The quantity requested is less than or equal to the program quantity
limit OR
ii. The quantity (dose) requested is greater than the maximum dose
recommended in FDA approved labeling and the prescriber has
submitted documentation in support of therapy with a higher dose for
the intended diagnosis which has been reviewed and approved by the
Clinical Review pharmacist.
Length of Approval: 12 months
Renewal Evaluation will be approved when BOTH of the following are met:
1.
The patient has been approved previously for ivacaftor through the Prime Therapeutics
PA process AND
2.
ONE of the following:
a. The quantity requested is less than or equal to the program quantity limit OR
b. The quantity (dose) requested is greater than the maximum dose
recommended in FDA approved labeling and the prescriber has submitted
documentation in support of therapy with a higher dose for the intended
diagnosis which has been reviewed and approved by the Clinical Review
pharmacist.
Length of Approval: 12 months
MN_CS_Kalydeco_PA_ProgSum_AR1112.doc
© Copyright 11/2012 All Rights Reserved
Page 3 of 3