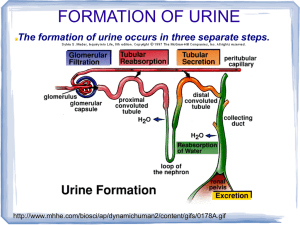
Introduction to the Composition of Seawater Water is essential to life
advertisement

Introduction to the Composition of Seawater Water is essential to life and central to our study of the oceans! It exists in many different places on Earth, some more accessible than others, and known collectively as reservoirs. The processes that transfer the water molecule – H2O – between reservoirs form the hydrologic cycle. List as many major reservoirs of water on Earth as you can. Example: oceans Values unless noted from Pinet’s Invitation to Oceanography Reservoir Percent of Earth’s Water at Surface Oceans 97.25 Ice caps and glaciers 2.05 Groundwater 0.68 Lakes and ponds 0.01 Atmosphere 0.001 Rivers 0.0001 Biosphere 0.00004 Soils 0.005 (from Berner and Berner, Global Environment, 1996) Total 99.99 (correct number of significant figures) Example of the hydrologic cycle. Items may be drawn individually as students contribute them. Which three reservoirs do you think contain the most water? See above. Of all the reservoirs you listed, in which do you predict a water molecule will have the longest average stay? The shortest? Shortest stay is in the atmosphere (days to weeks; ask students to think about what they know about storm systems). Longest stays in the near surface reservoirs will be ice caps (thousands to millions of years) and groundwater (days to thousands of years). List all the processes you know that transfer water molecules between reservoirs. evaporation; precipitation; subsurface flow/infiltration; runoff; evapotranspiration; sublimation; wind (vapor and condensed water); melting/runoff; hydrothermal cycling When you finish this page, stop. We’ll piece the information together at the board into an illustration of the hydrologic cycle, which we’ll need before answering the back. The dissolved substances in water are known as solutes. Some transport processes in the hydrologic cycle carry both solutes and water. Which of the transport processes in the hydrologic cycle are most likely to transport both solutes and water? Runoff and groundwater flow. Hydrothermal fluids will have the most concentrated solutes, but they are not typically included in the hydrologic cycle. Which transport processes in the hydrologic cycle are unlikely to transport large quantities of solutes? Evaporation; precipitation; sublimation; evapotranspiration; wind. (Sometimes students will think that evaporation transports solutes.) What types of substances, including solutes, are likely to be in seawater? Solutes: salts, gases (dissolved), nutrients, metals (dissolved), acids and bases (list of solutes will vary with students’ prior background. Some nutrients and metals can also be acids or bases; some metals are present as salt ions.) Also sediments, organisms, and water Which of the substance types (above) are solutes? See answer above. Place a star next to any of the types of substances that would also be found in fresh water, for example, a Maine pond. All of the above! The concentration of a substance in water is the amount (mass, volume, molecules, etc) of the substance present in a given amount of water (mass, volume, molecules, etc). For any substance present in both fresh water and seawater, predict where it will be more concentrated: More concentrated in seawater More concentrated in fresh water Salts; others highly variable gases (typically); nutrients (typically)