Chemistry Review Study Guide
advertisement

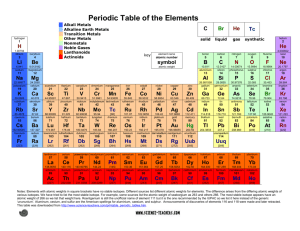
Chemistry Review Study Guide Define and explain: Element Group/family Compound Period Homogenous mixture Metal Solution Non metal Substance Metalloid Chemical property Transition metal Physical property Solution Nucleus Mixture Electron Solubility Neutron Saturated Proton Supersaturated Atomic cloud Oversaturated Atomic number Supersaturated Atomic mass Isotope Periodic table Be able to: Determine the number of protons, neutrons, electrons, atomic number and atomic mass Element Atomic # Oxygen Atomic Mass 16 Protons 8 Neutrons Electrons Read a solubility curve EXAMPLE: How many grams of KNO3 are needed to make a saturated solution of 100g of water at 59 degrees? Draw an atomic model o + Understand the layout of the period table o How are elements grouped? o Properties of metals and non metals and where they are places on the periodic table o Group # of : Noble Gas, halogens, alkali metals, alkaline metals, transitional metals Know the difference between element, compound and mixture