optical properties and stability of perovskite based
advertisement

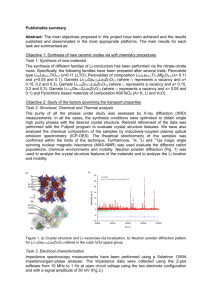
OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL A. 2014 The Effect of UV light Exposure on Perovskite based solar cell Mesoporous TiO2 have reached close to commercial power conversion efficiencies of 12.3% using a liquid electrolyte and up to 15% with solid state transporter and organometal halide perovskite absorber. This solid state approachs have the advantage that they do not suffer from electrolyte leakage. Nor does the hole-transporting material (HTM) posses any corrosive qualities that we know of. A critical challenge for global adoption of perovskite-based solar cell is the proof or realization of longetivity. In addition, ultraviolet (UV) cutoff filters are often used, but the effect of UV light on solar cell stability is not yet well understood. In contrast, a great deal of literature is available on the subject of long term stability of polymer –based solar cells, which require encapsulation to protect the organic component from oxidation in air. Air sensitivity of organics, coupled with moisture sensitivity of the current generation of perovskite absorbers, make it clear that this new class of solar cell will require a high level of encapsulation to attain eventual outdoor lifetimes of over 25 years. With that in mind, we have performed the series of aging tests on TiO2- SSCs using both dye-sensitizer and the methylammonium lead iodide chloride mixed halide erovskite absorber CH3NH3PbI3-xClx in different environments. The-TiO2-SSCs encapsulated in a nitrogen atmosphere suffer from an extremely rapid decay in photocurrent and performance under stimulated sunlight with the full UV component present. In this research, the writer describe the mechanism driving this drop in performance and propose that oxygen serves to pacify deep electronic trap sites at oxygen vacancies in TiO2. Indeed, that has benn demonstrated that the high efficiency MSSCs exhibit extremely stable photocurrent over 1000 hours of continuous full spectrum AM 1.5 solar illumination at 400C. this identify a key advantage of MSSCs, not only does it deliver fundamentally higher efficiency, but removal of the mesoporous TiO2 also sidesteps a critical stability. RESULT AND DISCUSSION To investigate the UV aging effect on the perovskite based solar cells, we have exposed a variety of different solar cell configurations to 5 hours of stimulated AM 1.5 1 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 100 mWcm-2. Figure 1 shows the evolution of solar cell performance parameters over time under illumination for TiO2-based solar perovskite SSCs, measured in air, encapsulated in nitrogen-filled glove box, and ecapsulated bt with the additional filtration of the UV component of the light by using 435-nm UV cutoff filter. Figure 1. aging of TiO2-based solar cells. Evolution of normalized solar cell performance parameters, power conversion efficiency, short circuit current (J sc), Fill factor (FF), and open circuit voltage (V oc) over 5h of AM 1.5 100mWcm-2 olar illumination. We compare the behavior f perovskite sensitized TiO 2 solar cells which are non-encapsulated (blue open circle) and encapsulated with (filled black square) and without a<435-m cutoff folter (open red circuit). We note that all devices had initial power conversion efficiency of at 5%, with short circuit current of at least 10 mAcm-2, representing typical working solar cells of this type. From the figure above shows surprisingly that the encapsulated sensitized TiO2 cells decay much faster than non-encapsuated solar cells when subjected to full spectrum sunlight. When UV filter (435-nm cutoff) is used, however, the solar cells are more stable. The fill factor drop may simply be due to non-optimum doping HTM. In an attept to determine whether the drop in photocurrent is caused by deterioration in charge generation or charge collection, we have performed simultaneous charge extraction (at short circuit) and transient absorption spectroscopy (TAS) on complete solar cells with semi-transparent electrodes (15 nm gold) using a 550 nm laser pulse as the excitation. Figure 3 shows the current extraction and transient absorption spectroscopy of TiO2-based solar cell. 2 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 The ratio between the charges generated (G) to those collected © is a direct measurement of the collection efficiency (C/Gx100%). In Fig. 3a shows the transient short-circuit photocurrent trace, and Fig 3b shows the transient absorption signal. The table inset to Fig. 3a gives the calculated values for G,C and charge collection efficiency. For this analysis, we assume that the number of holes in spiro-OMeTAD is equivalent to the number of electrons in the TiO2. To account for the lost charge after UV aging, have been proposed that the UVdegraded cells suffer from a deep trapping of injected electrons within newly available sites in TiO2 (Figure 3). The photocurrent extraction measurement suggest that these trapped electrons are immobile, while the TAS shows that they recombine with oxidized spiro-OMeTAD species on the μs-ms time scale. Figure 2. Current extraction and transient absorption spectroscopy of TiO2-based solar cells. In the top panel (a), the current collected from full perovskite-sensitized TiO2 solar cells with semitransparent electrode after 10 ns laser pulse (550 nm) is plotted against time after the excitation. Non-encapsulated (blue), encapsulated after ambient light exposure (grey) and encapsulated after UV aging (red) 5 min at 40mWcm-2, 365 nm are compared. The area underneath the curves represent the total amount of collected charge. In bottom panel (b), the transient absorption trace at short circuit of the hole on spiro-OMeTAD is probed at 510 nm, performed simultaneously with current extraction. The lines are merely guides to the eye. The table in Figure 3 shows the charge generated (G, estimated from the early time transient 3 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 absorption signal), collected (C, by integrating the current decay), and the ratio between the two as a percentage (C/G). the laser intensity was 80 μJ cm-2, giving charge densities similar to those found in devices under operating conditions. Figure 3. proposed mechanism for UV-induced degradation. Upon UV light exposure, the photogenerated holes react with the oxygen radicals adsorbed at surface oxygen vacancies (a,b). molecular oxygen is desorbed from hese sites, leaving unoccupied, deep surface traps sites and a free electron per site. These electrons will recombine with the excess of holes in the doped-hole transporter. Upon escitation of the sensitizer, eectronns are injected either (1) into the conduction band from wich they become deeply trapped or (2) directly into deep surface trap (c). These deeply trapped electrons are not mobile and recombine readily with the holes on the spiro-OMeTAD hole transporter (d). CONCLUSION Having established that TiO2-based solar cells have an inherent instability under UV illumination, there are several options to overcome this : Prevent the UV light from reaching the mesoporous TiO2 while still using it to generate photocurrent by UV filter Completely remove the mesoporous TiO2 Meso-superstructured perovskite solar cells have by passed a critical instability of the mesoporous TiO2-based solar cells, and show significant promise as large sclae, longterm energy source 4 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL B. 2014 Determining Perovskite Optical Properties Based on Its optical Absorption Edge Particularly striking are the remarkably high open circuit voltage, Voc (up to 1.13 V to date), that are obtained for iodide-based perovskite solar cells compared with their optical bandgap, Eg ( 1.57 eV). Quite general;y, the bandgap-voltage offset (Eg/q)-Voc is the useful measure to assess the electronic quality of solar absorber in a solar cell, including perovskites. His offset directly scales with detrimental recombination losses in the device, such as those via deep defects or interfaces. Further insight into the potential of a material as a photovoltaic absorber can be gined from its optical band edges and subbandgap absorption spectrum. Photohermal Deflection Spectroscopy (PDS) and Fouriertransform Photocurret Spectroscopy (FTPS) are appropriate methods to determine these optical properties. Moreover, it has been reported the effect of moisture ingress on the absorption spectrum. RESULT AND DISCUSSION The absorpance spectrum of an as-prepared CH3NH3PbI3 perovskite thin film on glass measured by PDS, is shown in Figure 1. Figure 1. PDS, FTSP, and 1-Rspecular - Tspecular spectra of CH3NH3PbI3 perovskite thin film as well as the PDS spectra of the glass substrate, all measured at room temperature. Film thickness d eff = 220 nm. 5 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 Figure 1 shows a remarkably abrupt absorptance onset at a photon energy of 1.5 eV. It also shows the spectrum of PDS measurement below the photon energy 1.5 eV (CH3NH3PbI3 band gap), is fully lmited by defects presenr in the substrate glass. To gain more information about sub-bandgap absorption of the CH3NH3PbI3 layer at photon energy below 1.5 eV, we performed FTPS measurement.there are several important points ensue from the FTPS spectrum of the CH3NH3PbI3 layer, shown in Fig 1. First, because of the nature of the characterization technique involved, this measuremet proves that the perovskite material has asuffeciently high photosensitivity to generate a photocurrent that is significant compared with its dark current. Second, the FTPS spectrum features the same abrupt onset and sharb bending of the CH3NH3PbI3 absorptance as the PDS. Third, the FTPS curve does not saturate for photon energies below 1.5 eV. We obtain further insight into the relevance of the absorption spectrum by plotting the effective absorption coefficient of CH3NH3PbI3, as shown in Fig 2. Figure 2. Effective absorption coefficient of a CH3NH3PbI3 perovskite thin film compared with other typical photovoltaic materials, including amorphous silicon (a-Si), GaAs, CIGS, CDTe, and crystalline silicon (c-Si), all measured at room temperature. For each material, we show the slope of the Urbach tail. For the clarity, the inset shows the data for c-Si down to low absorption values. From the Fig. 2 it is particularly remarkable that the sharp shoulder near its repored badgap of the perovskite absorber occurs at higher absorption coefficient than the most other absorber films. Below this shoulder, the absorption coefficient of the 6 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 perovskite follows a purely exponential trend without much deviation toward lower photon energy. The slope of this exponential part of the curve is so called Urbach energy.we see that the Urbach energy of the CH3NH3PbI3 is close to the values of GaAs, a monocrystalline direct bandgap semiconductor of very high electrical quality. The sharp Urbach energy is generally attributed to inherent undisorder structur of materials. Here is also identified the effect of moistre ingress on the absorptance performance of perovskite (Figre 3). In Figure 3, it shows the PDS spectra of CH3NH3PbI3 layers after exposure to ambient air with 30-40% relative humidity during 1-20 hours. The figure shows that the absorptance of CH3NH3PbI3 after 20 hour exposure is shifting to higher energy that is 2.5 eV, which 2.3 eV corresponds to the bandgap of PbI2. It may indicate that CH3NH3PbI3 can decompose into PbI2 in a humid ambient due to the dissolution of disordered CH3NH3PbI3. Figure 3. PDS spectra of CH3NH3PbI3 perovskite thin films during prolonged immersion I the FC-72 solution and following moisture exposure CONCLUSION The absorption coeficent of organometallic halide perovskite follows a purely exponential trend without much deviation toward lower photon energy The low Urbach energy measured for the CH3NH3PbI3 perovskite film suggests a very low degree of structural disorder and no optically detectable deep state are present 7 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 Both PDS and FTPS are the suited technique to observe the optical band-gap and sub bandgap absorption of organometallic halide perovskite Upon moisture ingress, the strong absorption onset suggests a compositional change of the CH3NH3PbI3 8 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL C. 2014 Perovskite-Based Monolithic Series Type and Its Properties A new promising class of light harvesting materials, namely the hybrid organic halide based perovskites have been recently employed to realize high efficiency photovoltaic solar cells. This kind of crystalline material shows good properties interms of light harvesting (high absorption in a broad regionof the visible spectrum) and electron and hole mobilities. A maximum power conversion efficiency (PCE) of 15% was reported using CH3NH3PbI3-sensitized TiO2 together with the Spiro-OMeTAD as HTM for small-area devices. Alternative HTMs have also been studied to replace the Spiro-OMeTAD in order to reduce the fabrication cost of scaled-up devices. Perovskite solar cells made with polymeric HTMs such as polytriarylamine (PTAA) and P3HT have shown a PCE of 12% and 6.7%, respectively. RESULT AND DISCUSSION Recently, we have reported the use of a regio-regular, doped, and high molecular weight P3HT in combination with CH3NH3PbI3-xClx showing a PCE of 9.3% for smallarea devices (0.1 cm2). However, scaling up to the module size is still an open issue for perovskite solar cell technology and reports on the feasibility of large-area devices are still missing. In this paper, the first perovskite-based monolithic series-type module was realised showing very promising results in terms of the power conversion efficiency, the reproducibility of the fabrication process and long-term stability. 9 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 Figure. 1 (a) Image of the monolithic perovskite-based module. (b) The monolithic interconnection scheme of the perovskite-based module. In particular, the length of the active area is equal to 48 mm, the width of each cell is 7 mm, the width of the etched FTO area is equal to 1 mm and the width of the contact area between the cells (where no c-TiO2 layer was deposited) is equal to 2.5 mm. Fig. 1a. Five single cells (active area of 3.36 cm2) are connected by using a monolithic scheme for series-interconnection with an overall active area of 16.8 cm2 and an aperture area of 25 cm2, as shown in Fig. 1b. The layout of the module allows the measurement of each cell individually. The I-V curve characteristics of the perovskite-based solar modules together with the I–V curve of the best cells using both HTMs comparing the I–V measurements of the best solar cell and the module for both HTMs is shown in Figure 2. Figure 2 (a) I–V characteristics of series-connected large-area P3HT-based devices and best cells are reported. (b) I V characteristics of series-connected large-area Spiro-OMeTAD-based devices and best cells. I–V characteristics are measured under standard illumination conditions AM1.5G (1000 W cm!2) under the class B Sun Simulator (solar constant 1200 KHS). The module with the P3HT HTM shows VOC = 4.5 V, ISC = -36.8 mA, FF = 52.6% and PCE = 5.1% on the active area. However, the module with spiro-OMeTAD HTM achieve a lower performance with VOC = 4.31 V, ISC = -32.9 mA, FF = 60.3% and PCE = 5.081% on the active area. 10 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 To observe the reproducibility of this module type, we check the photofoltaic performance of this module in the different scalig size as shown in Figure 3. Figure 3. Evolution of the PCE obtained during the up-scaling process using both HTMs: Spiro-OMeTAD (cyan colour) and P3HT (green colour) scaling the size of the active area (the small-area cell (0.1 cm2), the large-area cell (3.36 cm2) and the series-interconnected module (16.8 cm2)). The up-scaling process was carried out maintaining the same perovskite deposition procedure. Figure 3 shows the PCE values for different device sizes obtained maintaining the same experimental conditions for each device size. The results show that the PCE drops by about 30% migrating from the 0.1 cm2 cell to the about 40 times larger one (3.36 cm2) for both HTMs. This drop could be due to different factors including the resistivity of the FTO electrode and the non-uniform perovskite deposition on the large-area substrates using the spin coating technique. Upon scaling from the large-area cell to the module, the PCE drops by about 14% for Spiro based devices and 19% for P3HT-based devices. As previously reported, these slight decreases are due to the mismatch of ISC between the constituent cells forming the series-connected module. These results represent a significant starting point for future development of the perovskite-based modules. Subsequently, a light soaking ageing test for 335 hours wasperformed to evaluate the long-term stability of the perovskitebased module using both HTMs under AM1.5 illumination conditions (1 Sun at 40 1C illuminated by class A Light Soaker in air) as shown in Fig. 4a and b. 11 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 Figure 4. Evolution of normalized photovoltaic parameters (PCE, red squares; VOC, cyan circles; ISC, blue triangles; FF, green stars) using different HTMs under AM1.5G illumination conditions with a class A light soaker (Solaronix, SOLARTEST 65-STF65) at 1000 W m-2 and 40 0C in air. The encapsulated modules are maintained under VOC conditions during the light soaking test. (a) Long-term stability of the P3HT-based module after 170 hours. (b) Long term stability of the Spiro-based module after 335 hours. CONCLUSION To produce an efficient and reproducible up scealing process of hybrid organic halide perovskite-based devices. Here mentioned the key ones : The patterned deposition procedure for TiO2 layer The optimization of the monolithic interconnection between constituent cells The uniform deposition of thin titania scaffold by screen-printing Uniform deposition of large area CH3NH3PbI3-xClx deposition by spin coating 12 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL D. 2014 Effect of Annealing on Film Morphology of Perovskite Solar Cell There has been a recent surge of progress in the fi eld of thin fi lm solar cells focusing on the integration of three-dimensional organic-inorganic hybrid perovskites in solar cell devices. The solution processability of the organic-inorganic perovskite material is one of its major advantages, the simplest method of deposition requiring only a simple heating step to convert the deposited precursor solution (consisting of the organic and inorganic components) to the crystalline perovskite form. Here, we investigate the effect and importance of the annealing temperature during the final conversion step, relating it to the perovskite film morphology and its influence on the device working mechanisms. These perovskite films formed are analyzed using scanning electron microscopy (SEM) and X-ray diffraction (XRD) measurements. This exposes the role of the annealing temperature of the perovskite on its film composition and morphology. The variation of the annealing temperature during the perovskite conversion step was correlated with the photovoltaic performance of solidstate solar cells using spiro- MeOTAD as HTM. RESULT AND DISCUSSION The influence of the annealing temperature on the morphology and structure of the perovskite layer formed on the mTiO2 were examined by scanning electron microscopy (SEM). 13 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 Figure 1. SEM images of mTiO2 films with deposited perovskite solution heat treated at a) 60 °C, b) 80 °C, c) 100 °C, d) 120 °C, e) 150 °C, f) 175 °C, and g) 200 °C. h) Cross sectional image of sample annealed at 150 °C. Black scale bars correspond to a) 5 μm, b-g) 1 μm, and h) 200 nm. For annealing temperature of the 600C, there is notypically observed for such perovskite materials. As the annealing temperature is increased to 800C there is a drastic change in the appearance of the film formed. The typical structure of perovskite is visible. The film formed upon annealing at 100 0C is relatively similar in appearance to that formed at 80 0C. Increasing the annealing temperature to 120 0C, the morphology of the perovskite adopts larger individual size for the island material. As the annealing temperature is further increased , the size of perovskite island becomes larger and furthermore internal structuring becomes dicernable. X-ray diffraction measurements were conducted to investigate the nature of the material formed following the annealing process 14 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 Figure 2. X-ray diffraction patterns of perovskite samples annealed at different temperatures. The perovskite was deposited on meso-TiO2 coated onto FTO substrates. The vertical lines indicate the peaks associated with the perovskie material (stars), PbI 2 (triangles), PbCl2 (circles), and the FTO/mTiO2 substrate (dotted line) The XRD pattern for sample dried at ambient temperature for more than 20h exhibit a large number of peaks that are mainly attributed to the precursor materials. Once the annealing temperature is increased to 80 0C, the peaks for precursors are no longer present and only the peak of the perovskite materials remain. The XRD pattern for the sample annealed at 100 0C shows little different to the 80 0C sample. For the heat treatments at 200 0C the intensity of the perovskite is decreased and is explained by the decrease in aterial formed at these high temperature. The current voltage (J-V) characteristics measured under standart AM 1.5G illumination (100 mWcm-2) of these devices are presented in Fig. 3. 15 OPTICAL PROPERTIES AND STABILITY OF PEROVSKITE BASED SOLAR CELL 2014 A clear correlation is observed between the annealing temperature of the perovskite and the photovoltaic performance of the devices. Samples that were annealed at 100 0C gave the highest power conversion efficiency (PCE), 11.66%. at low annealing temperatre (60 0C) parameters are decreased as a consequence of incomplete conversion to the perovskite material, leading to lower light harvesting as there is unsufficient perovskite sensitizer present. The device annealed at 80 0C displayed high PDE, and generally their paraeers were only slightly lower than the maximum values observed at 100 0C CONCLUSION There is an energy requirement to the formation of the perovskite Low temperature (below 600C ) is insufficient to form the CH3NH3PbI3 perovskite. Too high annealing temperature (above 2000C) leads to the formation of PbI2 the highest efficiency is achieved by 1000C annealing temperature 16