final1-publishable-summary-final-apr-15
advertisement
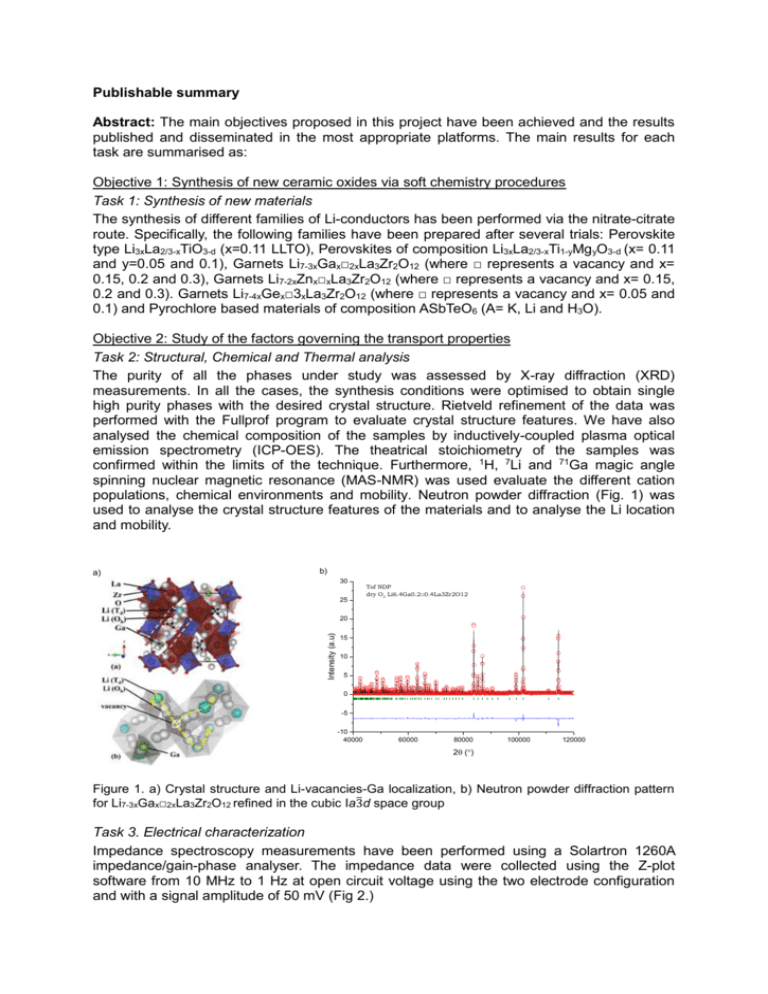
Publishable summary Abstract: The main objectives proposed in this project have been achieved and the results published and disseminated in the most appropriate platforms. The main results for each task are summarised as: Objective 1: Synthesis of new ceramic oxides via soft chemistry procedures Task 1: Synthesis of new materials The synthesis of different families of Li-conductors has been performed via the nitrate-citrate route. Specifically, the following families have been prepared after several trials: Perovskite type Li3xLa2/3-xTiO3-d (x=0.11 LLTO), Perovskites of composition Li3xLa2/3-xTi1-yMg yO3-d (x= 0.11 and y=0.05 and 0.1), Garnets Li7-3xGax□2xLa3Zr2O12 (where □ represents a vacancy and x= 0.15, 0.2 and 0.3), Garnets Li7-2xZnx□xLa3Zr2O12 (where □ represents a vacancy and x= 0.15, 0.2 and 0.3). Garnets Li7-4xGex□3xLa3Zr2O12 (where □ represents a vacancy and x= 0.05 and 0.1) and Pyrochlore based materials of composition ASbTeO6 (A= K, Li and H3O). Objective 2: Study of the factors governing the transport properties Task 2: Structural, Chemical and Thermal analysis The purity of all the phases under study was assessed by X-ray diffraction (XRD) measurements. In all the cases, the synthesis conditions were optimised to obtain single high purity phases with the desired crystal structure. Rietveld refinement of the data was performed with the Fullprof program to evaluate crystal structure features. We have also analysed the chemical composition of the samples by inductively-coupled plasma optical emission spectrometry (ICP-OES). The theatrical stoichiometry of the samples was confirmed within the limits of the technique. Furthermore, 1H, 7Li and 71Ga magic angle spinning nuclear magnetic resonance (MAS-NMR) was used evaluate the different cation populations, chemical environments and mobility. Neutron powder diffraction (Fig. 1) was used to analyse the crystal structure features of the materials and to analyse the Li location and mobility. a) b) 30 25 Tof NDP dry O2 Li6.4Ga0.2□0.4La3Zr2O12 Intensity (a.u) 20 15 10 5 0 -5 -10 40000 60000 80000 100000 120000 2 () Figure 1. a) Crystal structure and Li-vacancies-Ga localization, b) Neutron powder diffraction pattern for Li7-3xGax□2xLa3Zr2O12 refined in the cubic Ia3̅d space group Task 3. Electrical characterization Impedance spectroscopy measurements have been performed using a Solartron 1260A impedance/gain-phase analyser. The impedance data were collected using the Z-plot software from 10 MHz to 1 Hz at open circuit voltage using the two electrode configuration and with a signal amplitude of 50 mV (Fig 2.) d) a) b) c) Figure 2: a) Equivalent circuit used for the impedance data fitting. Nyquist plots of the LLTO samples sintered in different atmosphere: LLTO-A (squares), LLTO-SA (circles) and LLTO-O (triangles) b) obtained from 10 MHz to 7Hz and c) from 10MHz to 10 kHz. d) SEM images and particle size distribution of LLTO pellets sintered Task 4: Electron microscopy measurements and trace diffusion experiments Scanning Electron Microscopy (SEM – SEM Quanta 200 FEG) operated in low vacuum mode at a voltage of 20 kV was used to analyse the microstructure of the samples. The grain shape and boundaries were revealed by thermal etching. The grain size distribution was calculated with the software Estereologia from a 2500 m2 surface area (Fig. 2c). Task 5: Tracer ionic diffusion measurements In order to investigate the surface composition, the LLTO sample was analysed by low energy ion scattering (LEIS). Fig. 3 shows the LLTO surface spectrum obtained by 3 keV He+ scattering. The presence of the C peak after the oxygen plasma exposure suggests the formation of a carbonate layer on the surface, as this would not be removed during the cleaning procedure. A surface peak corresponding to La cations located on the A-site of the LLTO perovskite appears at 2213 eV, although most of the surface would be covered by the carbonate layer and other contaminants (e.g. Na), as indicated by the small surface peak. The Ti atoms are then located underneath the AO plane termination of the LLTO perovskite implying that both Li cation and cation vacancies might be exposed in the surface facilitating the ion-exchange. Figure 3. LEIS surface spectrum for 3 keV 4He+ ions scattered from the LLTO pellet. Dashed lines: background components corresponding to the secondary ions sputtered from the sample surface (grey line, exponential decay at low energies) and the atoms located at the sub-surface (green=Ti, blue=La). Inset: Surface spectra corresponding to the bulk composition after Ar + sputtering.