neuro 276 to 297[2-9
advertisement
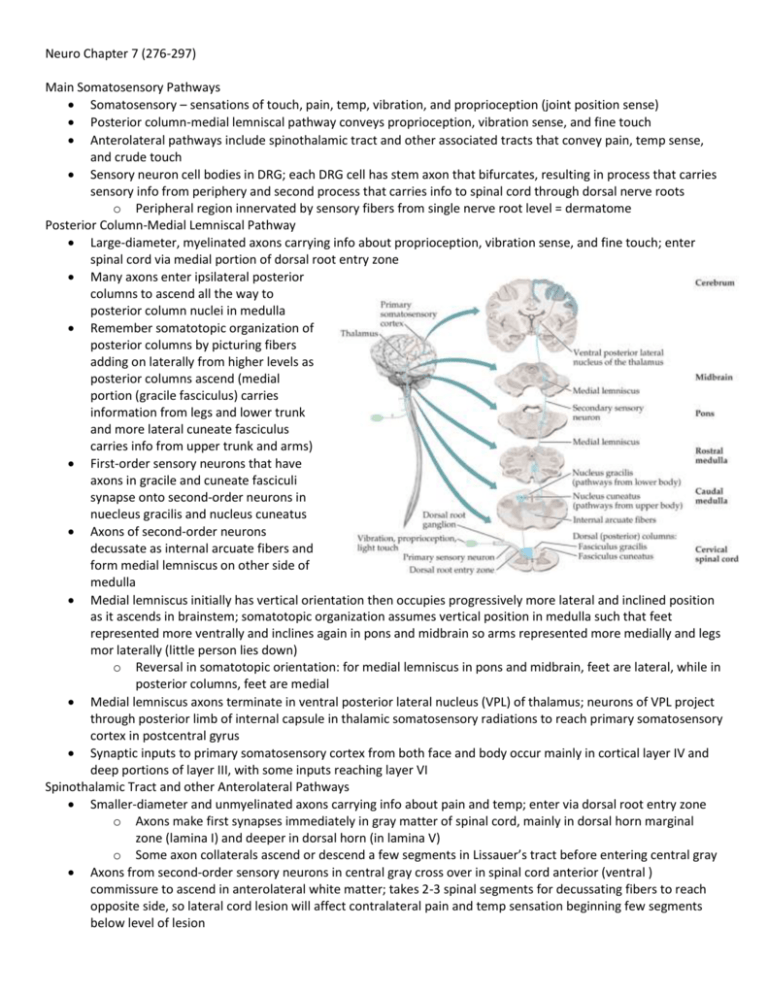
Neuro Chapter 7 (276-297) Main Somatosensory Pathways Somatosensory – sensations of touch, pain, temp, vibration, and proprioception (joint position sense) Posterior column-medial lemniscal pathway conveys proprioception, vibration sense, and fine touch Anterolateral pathways include spinothalamic tract and other associated tracts that convey pain, temp sense, and crude touch Sensory neuron cell bodies in DRG; each DRG cell has stem axon that bifurcates, resulting in process that carries sensory info from periphery and second process that carries info to spinal cord through dorsal nerve roots o Peripheral region innervated by sensory fibers from single nerve root level = dermatome Posterior Column-Medial Lemniscal Pathway Large-diameter, myelinated axons carrying info about proprioception, vibration sense, and fine touch; enter spinal cord via medial portion of dorsal root entry zone Many axons enter ipsilateral posterior columns to ascend all the way to posterior column nuclei in medulla Remember somatotopic organization of posterior columns by picturing fibers adding on laterally from higher levels as posterior columns ascend (medial portion (gracile fasciculus) carries information from legs and lower trunk and more lateral cuneate fasciculus carries info from upper trunk and arms) First-order sensory neurons that have axons in gracile and cuneate fasciculi synapse onto second-order neurons in nuecleus gracilis and nucleus cuneatus Axons of second-order neurons decussate as internal arcuate fibers and form medial lemniscus on other side of medulla Medial lemniscus initially has vertical orientation then occupies progressively more lateral and inclined position as it ascends in brainstem; somatotopic organization assumes vertical position in medulla such that feet represented more ventrally and inclines again in pons and midbrain so arms represented more medially and legs mor laterally (little person lies down) o Reversal in somatotopic orientation: for medial lemniscus in pons and midbrain, feet are lateral, while in posterior columns, feet are medial Medial lemniscus axons terminate in ventral posterior lateral nucleus (VPL) of thalamus; neurons of VPL project through posterior limb of internal capsule in thalamic somatosensory radiations to reach primary somatosensory cortex in postcentral gyrus Synaptic inputs to primary somatosensory cortex from both face and body occur mainly in cortical layer IV and deep portions of layer III, with some inputs reaching layer VI Spinothalamic Tract and other Anterolateral Pathways Smaller-diameter and unmyelinated axons carrying info about pain and temp; enter via dorsal root entry zone o Axons make first synapses immediately in gray matter of spinal cord, mainly in dorsal horn marginal zone (lamina I) and deeper in dorsal horn (in lamina V) o Some axon collaterals ascend or descend a few segments in Lissauer’s tract before entering central gray Axons from second-order sensory neurons in central gray cross over in spinal cord anterior (ventral ) commissure to ascend in anterolateral white matter; takes 2-3 spinal segments for decussating fibers to reach opposite side, so lateral cord lesion will affect contralateral pain and temp sensation beginning few segments below level of lesion Somatotopic organization with feet most laterally represented (fibers from anterior commissure add on medially as anterolateral pathways ascend in spinal cord) o When they reach medulla, anterolateral pathways located laterally, running in groove between inferior olives and inferior cerebellar peduncles; then enter pontine tegmentum to lie just lateral to medial lemniscus in pons and midbrain Anterolateral pathways consist of spinothalamic, spinoreticular, and spinomesencephalic tracts o Spinothalamic tract mediates discriminative aspects of pain and temp sensation such as location and intensity of stimulus Major relay for spinothalamic tract is ventral posterior lateral nucleus (VPL) of thalamus (separate neurons than from posterior column-medial lemniscal pathway) From VPL, info travelling in spinothalamic tract conveyed via thalamic somatosensory radiations to primary somatosensory cortex in post-central gyrus Pain and temp sensation for face carried by trigeminothalamic tract Spinoreticular tract terminates on medullary-pontine reticular formation, which in turn projects to intralaminar thalamic nuclei (centro-median nucleus); intralaminar nuclei project diffusely to entire cerebral cortex and involved in behavioral arousal Spinomesencephalic tract projects to midbrain periaqueductal gray matter and superior colliculi; periaqueductal gray participates in central modulation of pain Anterolateral pathways can convey some crude touch sensation If you step on a tack, spinothalamic tract makes you realize “something sharp is puncturing my foot”; spinothalamic intralaminar projections and spinoreticular tract say “ouch, that hurts”; spinomesencephalic tract leads to pain modulation to think “that feels better” later Somatosensory Cortex From thalamic VPL and VPM nuclei, somatosensory info conveyed to primary somatosensory cortex in postcentral gyrus; primary somatosensory cortex somatotopically organized with face most laterally and leg most medially Info from primary somatosensory cortex conveyed to secondary somatosensory association cortex in Sylvian fissure, along superior margin in parietal operculum region Further processing of somatosensory info occurs in association cortex of superior parietal lobule Primary somatosensory cortex and somatosensory association cortex have lots of connections with motor cortex Lesions of somatosensory cortex and adjacent regions produce cortical sensory loss Central Modulation of Pain Gate control theory – sensory inputs from large-diameter, nonpain A-β fibers reduce pain transmission through dorsal horn (why shaking your hand after hitting your thumb with hammer temporarily helps pain) Periaqueductal gray receives inputs from hypothalamus, amygdala, and cortex, and inhibits pain transmission in dorsal horn via relay in region at pontomedullary junction (rostral ventral medulla or RVM); includes serotonergic neurons of raphe nuclei that project to spinal cord, modulating pain in dorsal horn o RVM sends inputs mediated by substance P to locus ceruleus, which sends noradrenergic projections to modulate pain in spinal cord dorsal horn o Histamine contributes to modulation of pain through H3 receptors Opiate medications exert analgesic effects through receptors located on peripheral nerves and neurons in spinal dorsal horn; opiate receptors and endogenous opiate peptides found in high concentrations at key points in pain modulatory pathways; enkephalin-containing neurons and dynorphins-containing neurons concentrated in periaqueductal gray, RVM, and spinal cord dorsal horn; β-endorphin-containing neurons concentrated in regions of hypothalamus that project to periaqueductal gray Thalamus Nearly all pathways that project to cerebral cortex do so via synaptic relays in thalamus Thalamus also conveys nearly all other inputs to cortex, including motor inputs from cerebellum and basal ganglia, limbic inputs, widespread modulatory inputs involved in behavioral arousal and sleep-wake cycles, etc. Some thalamic nuclei have specific topographical projections to restricted cortical areas, while others project more diffusely; nuclei typically receive dense reciprocal feedback connections from cortical areas to which they project; corticothalamic projections outnumber thalamocortical projections Thalamus located in diencephalon with hypothalamus and epithalamus (contains habenula, parts of pretectum, and pineal body) Thalamus divided into medial nuclear group, lateral nuclear group, and anterior nuclear group by internal medullary lamina; nuclei located within internal medullary lamina itself called intralaminar nuclei o Midline thalamic nuclei lie adjacent to third ventricle, several of which are continuous with and functionally very similar to intralaminar nuclei o Thalamic reticular nucleus forms extensive but thin sheet enveloping lateral aspect of thalamus Most of thalamus made up of relay nuclei – receive inputs from numerous pathways and project to cortex o Projections to primary sensory and motor areas most localized; lie mainly in lateral thalamus o All sensory modalities (except smell) have specific relays in lateral thalamus o VPL and VPM project to primary somatosensory cortex o Visual info relayed in lateral geniculate nucleus (LGN) o Auditory info relayed in medial geniculate nucleus (MGN) [lateral light, medial music] o Motor pathways leaving cerebellum and basal ganglia have specific thalamic relays in ventral lateral (VL) nucleus en route to motor, premotor, and supplementary motor cortex Widely projecting (nonspecific) thalamic relay nuclei – visual and other sensory inputs to pulvinar relayed to large regions of parietal, temporal, and occipital association cortex involved in behavioral orientation toward relevant stimuli o Diffuse relays of limbic inputs and other info involved in cognitive functions occur in mediodorsal nucleus (MD) as well as midline and intralaminar thalamic nuclei MD serves as major thalamic relay for info traveling to frontal association cortex Intralaminar nuclei lie within internal medullary lamina; receive inputs from numerous pathways and have reciprocal connections with cortex o Main inputs and outputs from basal ganglia o Divided into caudal intralaminar nuclei (centromedian nucleus; involved mainly in basal ganglia circuitry) and rostral intralaminar nuclei (have input and output connections with basal ganglia) Rostral intralaminar nuclei relay inputs from ascending reticular activating systems (ARAS) to cortex, maintaining alert conscious state Reticular nucleus – only nucleus of thalamus that doesn’t project to cortex; receives inputs mainly from other thalamic nuclei and cortex and projects back to thalamus o Consists of almost pure population of inhibitory GABAergic neurons; regulates thalamic activity Paresthesias Lesions of posterior column-medial lemniscal pathways described as tingling numb sensation, feeling of tight band-like sensation around trunk or limbs, or sensation similar to gauze on fingers when trying to feel things In lesions of anterolateral pathways, often sharp, burning, or searing pain Lesions of parietal lobe or primary sensory cortex may cause contralateral numb tingling; can have pain Dejerine-Roussy syndrome – lesions of thalamus causing severe contralateral pain Lhermitte’s sign – electricity-like sensation running down back into extremities on neck flexion; lesion in C-spine Lesions of nerve roots produce radicular pain that radiates down limb in dermatomal distribution; accompanied by numbness and tingling; provoked by movements that stretch nerve root Peripheral nerve lesions cause pain, numbness, and tingling Dysesthesia – unpleasant, abnormal sensation Allodynia – painful sensations provoked by normally nonpainful stimuli Hyperpathia or hyperalgesia – enhanced pain to normally painful stimuli Spinal Cord Lesions In acute, severe lesions, often initially phase of spinal shock characterized by flaccid paralysis below lesion, loss of tendon reflexes, decreased SNS outflow to vascular smooth muscle causing moderately decreased BP, and absent sphincteric reflexes and tone o Spasticity and UMN signs develop over weeks to months o Some sphincteric and erectile reflexes may return, often without voluntary control o Acute traumatic spinal cord lesions may have improved outcome if treated within first 8 hours with high doses of steroids Chronic myelopathy (spinal cord dysfunction) often seen with degenerative disorders of spine; spine and nerve roots compressed, so combo of UMN and LMN signs mimicking motor neuron disease can happen If tumors treated with radiation after patient loses ambulation, 80% can’t get it back; reverse is true Infarction of spinal cord due to anterior spinal artery occlusion leads to anterior cord syndrome Patients with myelitis usually present with spinal cord dysfunction that develops relatively quickly o MRI often shows T2 bright areas, and CSF has elevated WBC (usually lymphocytic) Sensory Loss: Patterns and Localization Primary somatosensory cortex – deficit is contralateral to lesion; discriminative touch and joint position sense often most severely affected, but all modalities may be involved o Sometimes all primary modalities relatively spared, but cortical sensory loss present (decreased sterognosis (perceiving and understanding form of object by touch) and graphesthesia (ability to feel writing on skin) o Associated deficits from involvement of adjacent cortical areas may include UMN-type weakness, visual field deficits, or aphasia VPL, VPM, or thalamic somatosensory radiations – deficit contralateral to lesion; may be more noticeable in face, hand (lips and fingertips), and foot than trunk or proximal extremities o All sensory modalities may be involved, sometimes with no motor deficit o Larger lesions may be accompanied by hemiparesis or hemianopia caused by involvement of internal capsule; lateral geniculate; or optic radiations Lateral pons or lateral medulla – lesion involves anterolateral pathways and spinal trigeminal nucleus on same side; causes loss of pain and temperature sensation in body opposite lesion, and loss of pain and temp sensation in face on same side as lesion Medial medulla – lesion involves medial lemniscus, causing contralateral loss of vibration and joint position sense Spinal Cord Syndromes Transverse cord lesion – all motor and sensory pathways either partially or completely interrupted; often sensory level (diminished sensation in all dermatomes below level of lesion) Hemicord lesions (Brown-Séquard syndrome) – damage to lateral corticospinal tract causes ipsilateral UMN-type weakness; interruption of posterior columns causes ipsilateral loss of vibration and proprioception; interruption of anterolateral systems causes contralateral loss of pain and temp sensation Central cord syndrome – in small lesions, damage to spinothalamic fibers crossing in ventral commissure causes bilateral regions of suspended sensory loss to pain and temperature; produce cape distribution o With larger lesions, anterior horn cells damaged, producing LMN deficits at level of lesion o Because anterolateral pathways compressed from medial surface by large lesions, there may be near complete loss of pain and temp sensation except for sacral sparing Posterior cord syndrome – cause loss of vibration and proprioception below level of lesion; with larger lesions there may be encroachment on lateral corticospinal tracts, causing UMN-type weakness o Vitamin B12 deficiency and tabes dorsalis (tertiary syphilis) preferentially affect posterior cord Anterior cord syndrome – loss of pain and temp sensation below level of lesion; LMN weakness o With larger lesions, lateral corticospinal tracts may be involved, causing UMN signs o Incontinence common because descending pathways controlling sphincter function ventrally located Anatomy of Bowel, Bladder, and Sexual Function Sensory info from S2-S4 ascends to higher levels of nervous system through posterior and anterolateral columns Voluntary somatic motor fibers arise from anterior horn cells at S2-S4 to control pelvic floor muslces and from specialized sphincteromotor nucleus of Onuf at S2-S4 to control urethral and anal sphincters Pelvic PNS arise from sacral parasympathetic nuclei (S2-S4), and SNS arise from intermediolateral cell column (T11-L1) In general, for lesions to affect bowel, bladder, or sexual function, bilateral pathways must be involved Sense of bladder fullness reaches sensory cortex, and micturition initiated by descending pathways from medial frontal micturition centers that activate detrusor reflex o Detrusor reflex mediated by intrinsic spinal cord circuits and regulated by pontine micturition center o Reflex normally initiated by voluntary relaxation of external urethral sphincter, which triggers inhibition of SNS to bladder neck, causing it to relax, and activation of PNS, causing detrusor muscle contraction o Sensation of urine flow through urethra activates continued sphincter relaxation and detrusor contraction o When flow stops, urethral sphincters contract, triggering detrusor relaxation through urethral reflex o Flow can be interrupted at any time by voluntary closure of urethral sphincter, which triggers detrusor relaxation Lesions affecting bilateral medial frontal micturition centers result in reflex activation of pontine and spinal micturition centers when bladder full o Urine flow and bladder emptying normal, but no longer under voluntary control Lesions below pontine micturition center and above conus medullaris levels S2-S4 initially cause flaccid, acontractile (atonic) bladder, which usually evolves over weeks to months to hyperreflexic (spastic) bladder o When bladder atonic, reflex contractions of urethral sphincters persist, resulting in urinary retention and bladder distention; post-void residual volume increased o In hyperreflexic bladder, detrusor-sphincter dyssynergia often occurs, in which both detrusor and urethral sphincter tone increased in uncoordinated, at times antagonistic, fashion When involuntary reflex bladder contractions occur, there may be sense of urinary urgency or urge incontinence Often residual volume increases because of incomplete emptying, although volume smaller than with acontractile bladder Lesions of peripheral nerves or spinal cord at S2-S4 cause flaccid areflexic bladder or significantly impaired bladder contractility; result can be due to loss of PNS outflow to detrusor and/or loss of afferent sensory info from bladder and urethra o Overflow incontinence present o Urinary retention and incontinence can also be caused by prostatic hypertrophy, urethral strictures, etc. Neurogenic bladder – refers to flaccid or hyperreflexic bladder disorders of neurologic origin Fecal continence controlled by descending pathways originating mainly in medial frontal lobes o Anal sphincter closure maintained by internal anal sphincter innervated by sacral PNS and external anal sphincter innervated by pelvic nerves arising from Onuf’s nucleus (voluntary), and pelvic floor muscles innervated by sacral anterior horn cells o GI motility depends on PNS from S2-S4 for colorectal smooth muscle beyond splenic flexure o Fecal incontinence caused by diffuse cerebral or medial frontal lesions, spinal cord lesions, or lesions of sacral nerve roots or pelvic or pudendal nerves o In acute spinal cord lesions, anal sphincter completely flaccid with loss of sacral PNS outflow, causing severe constipation During sexual arousal, stimuli from sensory modalities with internal psychological factors result in activation of spinal cord autonomic pathways involved in sexual function o Sensation from genitalia conveyed by pudendal nerve, reaching S2-S4 o In female, secretion of lubricating mucus by Bartholin’s glands PNS mediated, and increased vaginal blood flow and secretions SNS mediated o o o In male, both PNS and SNS contribute to erection; ejaculation occurs through SNS mediated contraction of smooth muscle, causing emission of semen into urethra, followed by rhythmic reflex contractions of striated muscles (pelvic floor, urethral sphincter, bulbospongiosus, etc.) resulting in forceful expulsion In spinal cord lesions, reflex erection and ejaculation may still occur, but highly variable Peripheral nerve lesions, higher-order cortical lesions, medications, etc., can all contribute to sexual dysfunction