C.elegans-neuronal-l..
advertisement
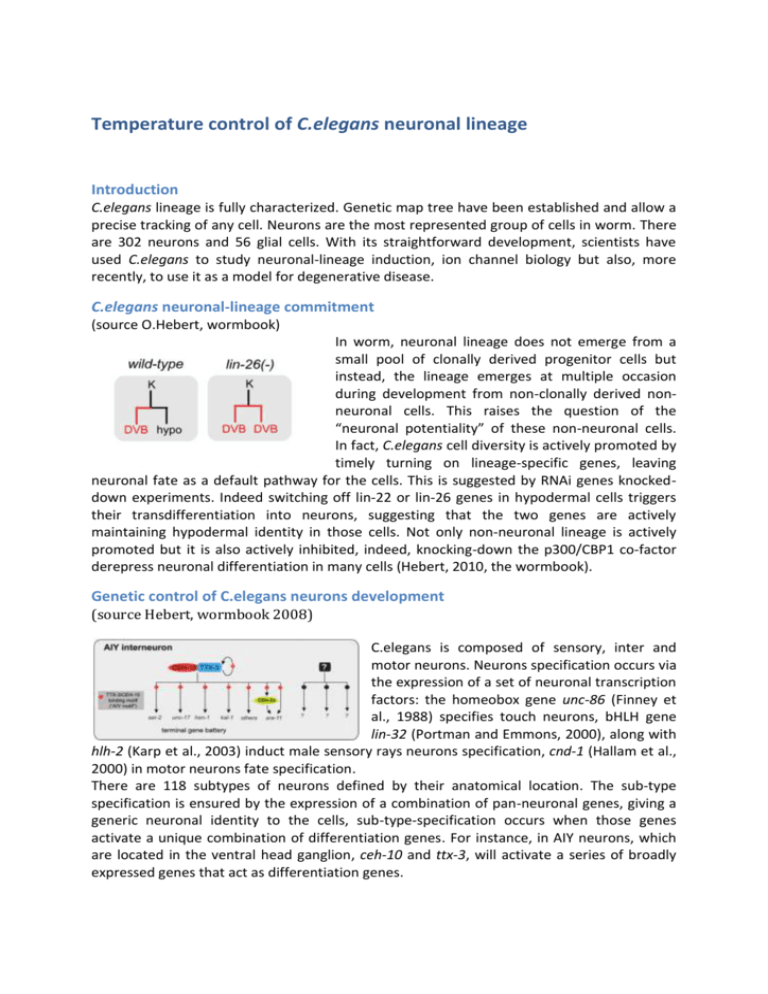
Temperature control of C.elegans neuronal lineage Introduction C.elegans lineage is fully characterized. Genetic map tree have been established and allow a precise tracking of any cell. Neurons are the most represented group of cells in worm. There are 302 neurons and 56 glial cells. With its straightforward development, scientists have used C.elegans to study neuronal-lineage induction, ion channel biology but also, more recently, to use it as a model for degenerative disease. C.elegans neuronal-lineage commitment (source O.Hebert, wormbook) In worm, neuronal lineage does not emerge from a small pool of clonally derived progenitor cells but instead, the lineage emerges at multiple occasion during development from non-clonally derived nonneuronal cells. This raises the question of the “neuronal potentiality” of these non-neuronal cells. In fact, C.elegans cell diversity is actively promoted by timely turning on lineage-specific genes, leaving neuronal fate as a default pathway for the cells. This is suggested by RNAi genes knockeddown experiments. Indeed switching off lin-22 or lin-26 genes in hypodermal cells triggers their transdifferentiation into neurons, suggesting that the two genes are actively maintaining hypodermal identity in those cells. Not only non-neuronal lineage is actively promoted but it is also actively inhibited, indeed, knocking-down the p300/CBP1 co-factor derepress neuronal differentiation in many cells (Hebert, 2010, the wormbook). Genetic control of C.elegans neurons development (source Hebert, wormbook 2008) C.elegans is composed of sensory, inter and motor neurons. Neurons specification occurs via the expression of a set of neuronal transcription factors: the homeobox gene unc-86 (Finney et al., 1988) specifies touch neurons, bHLH gene lin-32 (Portman and Emmons, 2000), along with hlh-2 (Karp et al., 2003) induct male sensory rays neurons specification, cnd-1 (Hallam et al., 2000) in motor neurons fate specification. There are 118 subtypes of neurons defined by their anatomical location. The sub-type specification is ensured by the expression of a combination of pan-neuronal genes, giving a generic neuronal identity to the cells, sub-type-specification occurs when those genes activate a unique combination of differentiation genes. For instance, in AIY neurons, which are located in the ventral head ganglion, ceh-10 and ttx-3, will activate a series of broadly expressed genes that act as differentiation genes. C.elegans as a model for neurodegenerative disease Source Oren-Suissa and Podbilewicz, Science 2010 Modeling neurodegenerative human disease in worm is not a trivial idea. Yet, bioinformatics analyses suggest that as much as 60 to 80% of C.elegans genes have homologs in human and with this respect worm can prove useful to screen for drugs and understand otherwise complex disease. C.elegans have been used to study Amyotrophic Lateral Sclerosis, a motor neuron degenerative disease caused by mutations in Sod1 gene. At the cellular level, Sod1 proteins misfold, aggregate in muscles and, indirectly, triggers motor neurons death. RI Morimoto’s found that expressing SOD1-YFP mutant proteins in a destabilizing temperature-sensitive mutant worms, aggravates the toxicity triggered by SOD1 mutant protein alone. This suggests that the level SOD1 triggered toxicity is also dependent on destabilizing genetic background (Gidalevitz et al., 2009). Using temperature-control to study C.elegans neuronal lineage and function Alzheimer, Parkinson, ALS (see above) and Huntington’s diseases are being studied in C. elegans (Li and Le, 2013). Both in ALS and Huntington’s diseases, it has been shown that temperature-sensitive mutant genes can act as modifiers to proteins misfolding and aggregation toxicity. Hence the possibility to combine live-cell animal imaging with rapid temperature shift from permissive to restrictive temperature is very important. With our temperature controller, you can watch GFP-tagged mutant protein aggregation concomitantly to the mutant phenotype induction. CherryTemp allows you to rapidly screen through temperature phenotype effects. With CherryTemp, you can shift from 5 to 45 C in seconds and uniquely test control and mutant conditions on the same worm. References O.Hobert, Specification of the nervous system, The C. elegans Research Community, WormBook, 2005 http://www.wormbook.org. M. Finney , G. Ruvkun, HR Horvitz, The C. elegans cell lineage and differentiation gene unc86 encodes a protein with a homeodomain and extended similarity to transcription factors, Cell,1988 D.S. Portman, S.W Emmons, The basic helix-loop-helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage, Development, 2000 S.Hallam, E. Singer, D. Waring, Y. Jin, The C. elegans NeuroD homolog cnd-1 functions in multiple aspects of motor neuron fate specification, Development, 2000 X. Karp, I. Greenwald, Post-transcriptional regulation of the E/Daughterless ortholog HLH-2, negative feedback, and birth order bias during the AC/VU decision in C. elegans, Genes Dev, 2003 T. Kaletta and M.O. Hengartner , Finding function in novel targets: C.elegans as a model organism, Nat Rev Drug Discovery, 2006 J. Li, W. Le, Modeling neurodegenerative diseases in Caenorhabditis elegans, Experimental Neurology, 2013 T. Gidalevitz, T. Krupinski, S. Garcia, RI. Morimoto, Destabilizing Protein Polymorphisms in the Genetic Background Direct Phenotypic Expression of Mutant SOD1 Toxicity, PLos Genetics, 2009