plastics and polymers
advertisement

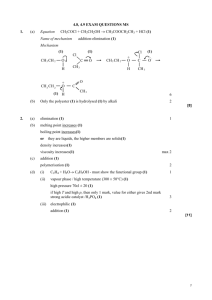
PLASTICS AND POLYMERS Chapter 12 TEFLON Describe the following terms: Macromolecule: A molecule that consists of a large number of atoms. Polymer: A large molecule composed of smaller monomer units covalently bonded to each other in a repeating pattern. Monomer: Small organic molecules that can be covalently bonded to each other in a repeating pattern. Polymerisation: A chemical reaction in which monomer molecules join to form a polymer. Addition polymerisation: A reaction in which small molecules join to form very large molecules by adding on double bonds. Addition polymer: A polymer formed when monomers combine through an addition reaction. Condensation polymerisation: Molecules of two monomers with different functional groups undergo condensation reactions with the loss of small molecules, usually water. Condensation polymer: A polymer formed by two monomers with different functional groups that are linked together in a condensation reaction in which a small molecule, usually water, is lost. Free radical: A molecular fragment with an unpaired electron. ADDITION POLYMERISATION This is done by joining together monomers which have C=C bonds to end up with long-chain molecules with C–C bonds. By changing the monomer one gets plastics with different properties. One can represent a polymer by abbreviating on a monomer level. For example Teflon (page 1) can be abbreviated as: It was made by adding together numerous tetrafluoroethene molecules. Addition polymerization occurs in a three step process as seen in this example of forming polythene (“polyethene”): 1. Initiation:- a free radical is generated which attacks a double bond of a monomer. (H2O2 is often used to generate free radicals.) R• + CH2=CH2 R–CH2–CH2• 2. Propagation:- the new free radical adds to another monomer creating a new longer free radical and this process continues creating a long chain – a polymer. R–CH2–CH2• + CH2=CH2 R–CH2–CH2–CH2–CH2• etc etc Until a long chain radical is formed…….. R–CH2–CH2– CH2–CH2– CH2–[CH2]n– CH2–CH2– CH2–CH2– CH2–CH2• 3. Termination:- two free radicals can join together to form an alkane: R–CH2–[CH2]n– CH2• + •CH2–[CH2]n– CH2–R R–CH2–[CH2]n– CH2–CH2–[CH2]n– CH2–R or a free radical can remove an H from another free radical to form an alkane and an alkene R–CH2–[CH2]n– CH2• + •CH2–CH2– CH2–R R–CH2–[CH2]n– CH3 + CH2=CH– CH2–R Polythene is the most common plastic and is used to make shopping bags, rulers, many containers and a myriad of other cheap items. CONDENSATION POLYMERIZATION This is done by having monomers which have functional groups which react together and in the process eliminate a small molecule. The most common example is the reaction between alcohols and carboxylic acids (both of which have functional groups at each end) to form esters – polyesters. H–O–CH2–[CH2]n–CH2–O–H + HOOC–[CH2]n–COOH O ǁ H–O–CH2– [CH2]n–CH2–O–OC–[CH2]n–COOH ester linkage Polyesters are used to make clothes, drinking bottles (PET)… Another famous example of a condensation polymer is Nylon. It is a polyamide. Properties of plastics 1. Light 2. Strong – the longer the molecules the stronger they tend to be. 3. Non-biodegradable – so do present a problem for disposal. Recycling has become more common to avoid this problem. 4. Insulators of heat and electricity. 5. Flexibility – this is determined primarily by the degree of cross-linking between chairs. The more cross-linking the more rigid the structure. Thermoplastics: Thermosets: Melt on heating and when cool they set in new shapes. e.g. polythene, nylon, polyvinyl chloride Break down at high temperatures so cannot be remoulded e.g. melamine Plastics and their uses. Plastic polythene nylon PET poly vinyl chloride PVC Teflon Kevlar Aramid poly(methyl methacrylate) PMMA Mylar Recycling – page 159 Uses bags, bottles rope, stockings water bottles pipes, flooring Non-stick body armour, tires. brakes windscreens Roasting bags ++