palm methyl ester
advertisement

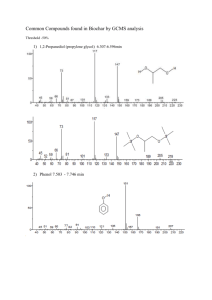
Conference Session #A12 Paper #2074 PALM METHYL ESTER: IMPROVING EMISSIONS AND REVOLUTIONIZING THE FUTURE OF FUELS Hannah Wardo (hbw@pitt.edu) and Gabrielle Campbell (gac31@pitt.edu) chemicals into the air which is commonly known as smog. Smog is particularly a problem in urban regions and has even caused many problems in air quality in the great city of Pittsburgh. In 2008 it was deemed as “America’s Most Polluted City” [3]. Smog is a mixture of greenhouse gases. Figure 1 below lists the greenhouse gases. Abstract- As the years progress and fossil fuel supplies deplete, we are faced with the need for an alternative source of fuel. Viable alternatives to fossil fuels are biodiesels, specifically the biodiesel palm methyl ester. Palm methyl ester is currently being produced and further developed by creating and testing new methods of production. This paper will focus on palm methyl ester by describing new methods of production for this biodiesel as well as comparing the new methods of production to the old methods. The effects that palm methyl ester has on engines and the environment will also be included. Information will be provided on the usage of this biodiesel in various countries, focusing on Malaysia, due to their large involvement in palm oil-based research and development [1]. In addition this paper will look towards the future and question whether this form of biodiesel could someday be used as regularly as fossil fuels are today. The paper will examine the high production costs of palm methyl ester and see if the new methods being researched can be used to reduce the cost of production to make this new alternative profitable. FIGURE 1 GREENHOUSE GASES [2] Key Words- Batch Processing, Continuous Production, Diesel engine, Malaysia, Palm Methyl Ester (PME), Transesterification, Nitrogen oxide is the major byproduct of combustion of machines. This compound can eventually turn into nitrogen dioxide then to acid rain. In addition nitrogen dioxide (NOx) reacts with highly reactive organic compounds found in the air to create ozone. Ozone is one of the most harmful pollutants and can have damaging effects on the environment [2]. The need for an alternative to fossil fuels has led to intensive research on biodiesels, one of these biodiesels being Palm methyl ester. Palm methyl ester has been recently researched and has presented data that proves palm methyl ester can be an effective alternative fuel. THE NEED FOR AN ALTERNATIVE FUEL As gas prices continue to rise and pollution becomes more of a problem for the heath of human beings and the environment, the need for an alternative energy source becomes a major issue. The main energy sources used at this time are fossil fuels which occupy almost 80 percent of all the energy used globally [2]. Although there is some evidence of fossil fuels improving people’s lives there is more evidence of fossil fuels ruining and hurting people’s lives. The process of retrieving the fossil fuels from the ground causes many problems to people’s well-being and their environment. Some of the causes of the problems include but are not limited to oil spills; transportation, which requires even more fossil fuels; and accidents on the extraction sites. It is proven that the drilling of oil causes the sinking of bodies of water and provokes earthquakes and eruptions of mud volcanoes near the drilling sites. Emissions from machines that use fossil fuels create a huge problem for human life and the environment. One of these problems is that combustion outputs a mixture of harmful WHAT IS PALM METHYL ESTER? Palm methyl ester (PME) is a biodiesel produced from esterification of crude palm oil and the mixing of methanol. For one mole of methanol, three moles of palm oil are added to produce palm methyl ester [4]. The chemical formula for Palm Methyl Ester can be seen below. R-COO-CH3 Palm methyl ester is the most promising alternative to diesel fuel because it is the most similar to diesel fuel, but has more environmental benefits and requires no modifications on diesel engines. Palm oil is also a likely Swanson School of Engineering April 14, 2012 University of Pittsburgh Twelfth Annual Freshmen Conference 1 Hannah Wardo Gabrielle Campbell alternative because palm trees have a very high oil yield compared to other oil producing crops. A country that has recognized its encouraging future in the biodiesel sector is Malaysia. type of biofuel. It required the mandatory mixing of this biofuel with regular petroleum diesel for industries. In addition it required all oil palm producing plants to have a license to do so. Eventually the licensing process was taken over by the International Trade and Industry. Unlike the International Trade and Industry group, the Malaysian Biodiesel Association is the only agency that is interested in the well-being and future developments in the biodiesel industry. The Malaysia Biodiesel Association has been pushing for a nationwide B5 mandate on commercial palm methyl ester. B5 mandate is the mandatory blending of five percent palm methyl ester with 95 percent petroleum diesel [1]. However, the cost of crude palm oil is currently too high to make the production of biodiesels profitable. This causes the mandate to be constantly pushed back until it is shown to be comparable to petroleum diesel in price. A way of lowering the price of palm methyl ester is to research and develop new ways of production. The current process being used is batch transesterification, and presently there is thorough research being done to improve this process, possibly through continuous production. Malaysia: The Growing Power behind Palm Methyl Ester Malaysia’s large oil palm cultivation covers 14% of the total area of their country. This allows Malaysia to be the world’s largest exporter and second largest producer of crude palm oil [1]. Due to their large amount of crude palm oil they have invested much time and research into developing this biodiesel further. A summarized timeline of Malaysia’s advancements with biodiesel can be seen in Figure 2. BATCH TRANSESTERIFICATION Palm methyl ester is currently produced by a process known as one or two- step batch transesterification [5]. Transesterification is an equilibrium reaction where an ester is produced into another ester through the exchange of an alkoxy moiety (-OR’) [5]. This leads to creating monoalkyl esters of vegetable oils; in this case, it leads to producing monoalky esters of palm oil. The reaction rate is increased by the addition of an acid or base catalyst. There are various types of catalysts used in transesterification. Acidic Catalyzed Batch Tansesterification The first type is an acidic catalyst. The advantage to this catalyst is that it outputs a high yield of biodiesel and the disadvantage to this catalyst is that it is very costly and is slower than the alkaline catalyst method, with reaction times ranging from three to 48 hours [6]. This type of transesterification requires a high alcohol to oil molar ratio to obtain high percent yields of the products. FIGURE 2 MALAYSIA’S TIMELINE OF PME DEVELOPMENT [1] In 2006 Malaysia launched the “National Biofuel Policy” which was supposed to bring long term benefits to the country and its biofuel industry. Some of the benefits included reducing the usage and dependency on fossil fuels, creating a stable price for crude palm oil, reducing greenhouse emissions, and making the palm oil industry boom. Following this national policy, Malaysia implemented “The Malaysian Biofuel Industry Act” which had a main goal to help further development in the biofuel industry. The act regulated palm methyl ester and no other Alkaline Catalyzed Batch Transesterification The second type of catalyst is an alkaline catalyst which has a 99 percent yield of biodiesel after only two hours of reaction. The most common alkaline catalyst is sodium and potassium hydroxide. The transesterification process while using an alkaline catalyst begins when the catalyst is dissolved into an alcohol, which is methanol, by continuous stirring in a small reactor vessel. Then the palm oil is 2 Hannah Wardo Gabrielle Campbell mixed with the combined methanol-alkaline catalyst mixture. Finally, the mixture is stirred forcefully for two hours. One disadvantage to these catalysts is that there is a formation of calcium foam at the initial stage of transesterification. This calcium foam reduces the yield of the biodiesel and makes it very difficult for product separation between the ester and the glycerol [7]. Figure 3 shows the batch transesterification process when a basic alkaline process is used. alkaline catalyst reactions, which have FFA interference. This interference is what leads to the calcium build up that makes it hard to obtain the produced palm methyl ester in the solution. The Downfalls of Batch Transesterfication FIGURE 3 BATCH TRANSESTERIFICATION USING AN ALKALINE CATALYST [8] CONTINUOUS PRODUCTION: AN ALTERNATIVE Batch transesterification has numerous downfalls which have led to more developments for continuous processing. A downfall of this reaction is that since it’s an equilibrium process and a reversible reaction only a certain amount of the product can be made without removing the product made before that batch. Also batch processes require large reactor volumes which lead to a high cost of raw materials needed to create the reactors. In turn this causes a domino effect and everything associated with this process also rises in price. Plus these large reactor volumes require large startup and shut-down energy expenditures which lead to major deviations in the efficiency of this process. A significant downfall to batch transesterification is that it is only meant for small scale production where they do not work around the clock. If palm methyl ester is to compete with diesel fuel it must be able to be produced at high volumes, which batch transesterifcation cannot provide. However, continuous production can. The last type of catalyst is the lipase based catalyst, also referred to as an enzyme catalyst, which yields 95% of biodiesel after 105 hours of reaction. Although these catalysts are very slow and more expensive, these catalysts have the benefit of allowing the reaction to proceed at room temperature. Another benefit is that glycerol produced using this method can easily be obtained. It is obvious that improvements have to be made to the batch transesterifcation way of producing palm methyl ester for it to be cost efficient and be competitive with petroleum diesel. Continuous production could be an alternative to batch transesterification. Developed in the early 1990s in Germany, specifically for biodiesel production, continuous processing allows companies on an industrial scale to produce biodiesel. Continuous production is a process that is operated 24 hours a day, and seven days a week. Semiannual or annual inspections are usually performed, which is usually the only day that production is shut down. Overall Analysis on Catalyzed Batch Transesterification Continuous Transesterification Through evidence it has been shown that alkaline catalyst are the best catalysts to use at this time due to the short amount of time needed to complete the process and the cheapness of the catalyst used in this type of transesterification. However, if more research was involved in trying to better the ways of producing palm methyl ester, there could be advancements in the other forms of transesterification, such as transesterification using acidic catalysts. If it was possible to lower the cost of acidic catalyst, or to develop a cheaper acidic catalyst, then it would be a better method of producing palm methyl ester and in general any type of biodiesel. This is because if an acidic catalyst is used, free fatty acids, also known as FFAs, do not interfere with the overall reactions, unlike the In 2000 the first research of its kind was done to develop a method of continuous transesterification of palm oil to produce palm methyl ester. This was done by using a continuous stirred-tank reactor (CSTR) and pumps, which supplied the palm oil and catalyst and also removed the produced palm methyl ester. If continuous transesterification was used to produce palm methyl ester, there would be many benefits. This includes the reduction in the size of the reactor volume and the reduction of the cost. It is also easier to control product quality and has more consistency in the created product. Lipase Based Catalyzed Batch Transesterification 3 Hannah Wardo Gabrielle Campbell membrane; the highest yield for palm methyl ester using this reactor was 95 percent [9]. Improving continuous transesterification using these methods could help launch the production of palm methyl ester into a larger scale. By having a larger scale of production of palm methyl ester while decreasing the cost of production could help make PME a competitive fuel option. Ways to Improve Continuous Transesterification Production If continuous transesterification production is improved, it could lead to a way for biofuels such as palm methyl ester to be produced on a large scale, making it easier to compete with petroleum diesel. Research has been done more recently on ways to improve continuous transesterification. Some of the most promising research was conducted in 2010 on modifications to the reactors and mixers used in the continuous production process. PETROLEUM DIESEL VS. PME A major benefit of Palm Methyl Ester is that is essentially requires very little to no modifications on the diesel engine. In addition, PME has been shown to obtain the highest energy content per volume compared to that of other alternatives fuels. Studies have been thoroughly conducted to prove why PME is a viable alternative based on these benefits. Ways to Improve Continuous Transesterification Processing: Micro-Channel Reactors The first modification was to replace the normal reactors with micro-channel reactors. Micro-channel reactors improve upon the efficiency of heat and mass transfer, by utilizing high surface area/ volume ratio and short diffusion distances [9]. The specific type of continuous transesterification studied using micro-channel reactors was transesterification using an alkaline based catalyst, which is the most commonly used type of transesterification both in batch and continuous processes. When a zigzag microchannel reactor was used at a temperature of 56 degrees Celsius and used for 28 seconds the yield of palm methyl ester was recorded to reach 99.5 percent [9]. When compared to the original reactor used in the continuous process the use of the micro-channel reactor led to less energy being used. Light-Duty Diesel Engines with PME A study was done in 2011 on replacing diesel fuel with blends of diesel fuel and palm methyl ester in light-duty diesel engines. A single-cylinder, naturally aspirated, fourstroke direct injection 347 cc diesel test engine was used to conduct the experiments [10]. The experiments were divided into two sections which were observing engine operational speed and determining emissions. The experiment involved observing engine operation speed when three types of fuels were used. These fuels included fossil diesel fuel, 100 percent PME, and B50, which is a mixture of half PME and half diesel. All three of these fuels were tested under various conditions of speed and weight exerted on the engine. Responses from the engine that were carefully examined were exhaust temperature, specific fuel consumption, efficiency of the charge input and output of the cylinders, the molar fuel to air ratio, exhaust gas emissions and cloudiness of smoke emitted. The results of the first section of the experiment when comparing 100 percent palm methyl ester to fossil diesel concludes that the three types of fuel used perform at similar trends. In all three types of fuel nitric oxide was shown to increase with speed as more fuel was burned. The general trend is that more time for combustion is given when a maximum temperature is used and the engine sustains a high load while performing at low speeds. The results for volumetric efficiency show that as engine speed increases volumetric efficiency weakens because a shorter amount of time is available to get air to the engine. One test that shows that palm methyl ester is a greener alternative to conventional diesel fuel was the smoke opacity test or the test to determine the cloudiness of the smoke emitted. It shows that the smoke opacity level for diesel fuel is much higher than that of fuel using palm methyl ester. Figure 4 illustrates these results. Other Reactors Which Improve Continuous Transesterification Another modified reactor that was tested was an oscillatory reactor. This reactor improved the mixing process by refining radical mixing and the transport process. This was done by both independently and controlled oscillatory motion. It was recorded that the conversion of palm oil to palm methyl ester in 99 percent concentration took approximately 30 minutes at 50 degrees Celsius [8]. This was done using an alkaline based catalyst transesterification process. One of the huge benefits of this type of reactor is that it significantly lowers the molar ratio of methanol to oil. Cavitational reactors were also shown to improve the process of continuous production by using the collapse of a cavity or the collapse of bubbles. This in turn produces high temperatures, pressures, and turbulence leading to a fast reaction rate. Microwave reactors also obtained a high reaction rate. The higher reaction rate occurred because it replaces the normal thermal heating being used with the heat caused by microwave radioactivity. Another reactor that achieved a high reaction rate was a membrane reactor. This was done by selectively removing glycerin by using a 4 Hannah Wardo Gabrielle Campbell fossil diesel in both pure and blended forms. In light-duty diesel engines palm methyl ester essential showed the same results in performance as fossil diesel with improvements in emissions without modifications to the engine itself. PME in Combustion Experiments Studies have also been conducted on the use of 100 percent palm methyl ester in gas turbines. In this study a comparison of 100 percent palm methyl ester and fossil diesel is performed by analyzing the combustion of the two fuels. A combustion chamber, air supply line, fuel supply line, and an exhaust gas analyzer were used to perform the experiments [4]. The temperature of the fuel that was supplied to the combustion chamber was regulated by an electric heater and the concentrations of the CO, NOx, and O2 were measured by the gas analyzer. The soot was measured by a smoke tester that was connected to the combustion chamber. The fuel temperature for palm methyl ester was set at 325 K and 303 K for diesel fuel for all experiments except for the experiment involving nitrogen dioxide emissions as a function of fuel kinematic viscosity, which is ratio of absolute viscosity to density [4]. A combustion experiment was performed to compare the ease of combusting palm methyl ester compared to that of fossil biodiesel. When this experiment was performed it was observed that there were no differences in the ignition of palm methyl ester, performing at the same level as diesel fuel. During this experiment it was observed that palm methyl ester did not accumulate soot while diesel fuel did. This occurs because palm methyl ester has no aromatic ring, but contains a large amount of oxygen. When emission tests were done it was observed that palm methyl ester and diesel fuel follow the same trend which is that NOx emission levels increase with a decreasing atomizing pressure. However, palm methyl ester has lower NOx emissions than diesel fuel at every air ratio measured. Figure 6 shows these trends. This is also true for kinematic viscosity. This is because the average particle size decreases with decreasing kinematic viscosity when a pressure-type atomizer is used [4]. FIGURE 4 SMOKE OPACITY LEVELS [10] In the second section of the experiment fossil fuel diesel, 100 percent PME, and eleven blends of diesel and PME in 10 percent intervals were used. A modified European Stationary Cycle (ESC) was used to test pollutant emissions, not only at non-idle positions, but at idle positions too. Results obtained from the second section of the experiment conclude that fuel blended with palm methyl ester had an overall reduction of the tailpipe emissions. The overall reduction of nitric oxide emissions showed a 5 percent drop of nitric oxide comparing 100 percent PME to fossil diesel. Results also show that there is a 26.2 percent lower concentration of unburned hydrocarbons when comparing 100 percent palm methyl ester to fossil diesel and only a .89 percent difference CO concentration found between 100 percent palm methyl ester and fossil diesel. When all four emissions test are compared it can be seen that smoke opacity levels shows the biggest support of palm methyl ester as an alternative. It shows that when 100 percent palm methyl ester is compared to fossil diesel there is a 66.7 percent reduction due to the amount of oxygen present in palm methyl ester and its impurity levels. Figure 5 illustrates the reduction of smoke opacity. FIGURE 5 SMOKE OPACITY LEVELS AS BIODIESEL CONTENT INCREASES [10] In conclusion, the experiments performed provide strong evidence as to why palm methyl ester is a good alternative to FIGURE 6 DECREASING NOX LEVELS [10] 5 Hannah Wardo Gabrielle Campbell From these experiments it can be concluded once again that palm methyl ester is a very promising alternative fuel for turbines. This is due to palm methyl ester having around the same efficiency as diesel fuel, which for both was nearly 100 percent in all cases. This research also shows that palm methyl ester as a fuel has lower NOx emissions. With lower NOx emissions, palm methyl ester is a more environmentally friendly fuel than diesel fuel. nationwide in 2014. The reason for the long time between introducing and implementing the mandate is because Malaysia needs to allow more time to get more biodiesel plants up and running. It is reported that once the mandate is set in place palm methyl ester will cost the same amount as petroleum diesel. However, the future of Malysia’s overall biodiesel industry strictly depends on whether or not the government provides incentives and subsidies for the industry. Some suspect that the B5 implementation will likely not have enough of a demand to keep the industry going [1]. The main reason for the lack of demand would be the cost of palm methyl ester overall which is caused by three major factors: cost of crude palm oil, selling price of biodiesel, and the cost of production of biodiesel. If petroleum diesel prices continue to rise, like they are now, then this biodiesel can become competitive with petroleum diesel in the energy industry. When the prices are competitive then a biodiesel, palm methyl ester, can be available which costs the same or even less than petroleum diesel but with many more benefits. THE POSITIVE ATTRIBUTES OF PME Palm methyl ester is considered to be one of the most environmentally friendly sources of fuel due to its reduction in emissions of greenhouse gases and NOx. The studies described above provide evidence on the reduction of these emissions compared to the emissions found by diesel fuel. By reducing NOx, the amount of ground ozone that is harmful to animals and humans will be reduced. Reducing NOx also will reduce the occurrence of acidic rain. Palm methyl ester as a biofuel is biodegradable with an expectancy to degrade 98 percent within three weeks whereas diesel fuel only degrades 50 percent in three weeks. This type of biofuel can also be used as a renewable energy, which can be seen in Figure 7. The production and consumption of Palm Methyl Ester can be described as a closed carbon cycle. This cycle begins with palm trees which then eventually produce palm biodiesel. Once it is a biodiesel it is consumed by vehicles which release carbon dioxide into the air. Carbon dioxide is then absorbed by palm trees making the total amount of carbon added by palm biodiesel zero. Palm Methyl Ester’s Future in Other Asian Countries Malaysia isn’t the only country taking large strides in the biodiesel industry. Other counties that use palm methyl ester as the main biodiesel are Indonesia, Korea, and Thailand. Indonesia requires a mixing of 10 percent PME with petroleum diesel, which is the highest mandate in any country as of right now. The biodiesel industry is expected to grow because of its strong energy policy which includes biofuels as a renewable energy source and not only wind and hydraulic energy. Indonesia expects its mandate to increase to a 20 percent blending by 2025 [12]. Korea imports palm methyl ester. Their biodiesel involvement began in 2007 with a B0.5 voluntary blending and every year since then has increased the blending by 0.5. In 2010 they reached the B2 blending which was mandatory. As of 2012 they are still on the B2 blending but are working this year to make the voluntary blending now a mandate. Korea’s goal for the blending of biodiesel and petroleum diesel is B20. They look to fulfill this goal by gradually increasing the blending by 0.5 every year or so [12]. In addition, in Korea, there are many taxes on petroleum diesel such as a traffic tax, an education tax, a driving tax and a ten percent value added price tax overall on the fuel. Consequently, biodiesels in this country are on only taxed with the ten percent value added tax. Thailand currently requires a B2 blending of palm methyl ester and fossil fuel diesel. In 2012 Thailand plans to reduce overall diesel consumption in their country by 10 percent. The National Energy Policy Council has come up with ways to promote usage of biodiesel. This council has provided incentives for companies wishing to produce blends of palm methyl ester. These incentives include a tax and payment exemption for the purchase of pure palm methyl ester. They FIGURE 7 PME POTENTIAL RENEWABLE ENERGY SOURCES [11] A FUTURE OUTLOOK FOR PME Malaysia is taking the biggest steps in the palm methyl industry for the future. They introduced a B5 mandate in 2008 which shows promise of being implemented 6 Hannah Wardo Gabrielle Campbell continuous biodiesel production.” Chemical Engineering and Processing: Process Intensification. [Online]’ Available: http://www.sciencedirect.com/science/article/pii/S0255270110000589 [10] J. Ng, H. Ng, and S. Gan. (2011) “Characterization of engine-out responses from a light-duty diesel engine fuelled with palm methyl ester (PME).” Applied Energy. [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0306261911000468 [11] “Outlook of Palm Biodiesel in Malaysia.” [Online]. Available: http://www.unapcaem.org/Activities%20Files/A0801/0202.pdf [12] M. Oguma, Y.J. Lee, and S. Goto. (2012) [Online]. Available: http://www.springerlink.com/content/734h22hg18j31524/fulltext.pdf also plan on implementing a B5 mandate, which will actually cost less than petroleum diesel. The reduced cost is because in this country palm methyl ester is essentially tax free. By 2022 Thailand plans to use 4.5 million liters per day of biodiesel, specifically palm methyl ester [12]. NATURE’S GIFT TO THE WORLD: PALM METHYL ESTER While palm methyl ester isn’t economically efficient, this biodiesel is well worth the effort to put more time and research into it to make it more cost efficient. Proven through research, palm methyl ester is confirmed to require essentially no modifications on the ordinary diesel engine while showing more benefits than petroleum diesel. It performs at the same level as petroleum diesel in terms of performance and also benefits the environment by showing less harmful emissions. Malaysia is the country that is most interested in this biodiesel realizing palm methyl ester’s full potential. Countries surrounding Malaysia have caught onto the idea of using palm methyl ester; however, no significant developments in this biodiesel have been shown in the United States. Although the United States has shown no significant developments in palm methyl ester, they have launched a program called twenty in ten which means they want to achieve twenty percent displacement of petroleum diesel in ten years. If the United States used its scientific resources to help in the further developments of palm methyl ester then there’s a possibility that a solution could be made to help this biodiesel become more cost efficient. By allowing the presence of palm methyl ester in the United States, it could lead to an overall greener world. ADDITIONAL RESOURCES [1] D. Darnoko and M. Cheryan. (2000) “Continuous Production of Palm Methyl Esters.” JAOCS. [Online]. Available: http://www.springerlink.com/content/m16180t27636tl3q/ [2] G. Pahl. (2005). Biodiesel: Growing a New Energy Economy. White River Junction, Vermont: Chelsea Green Publishing Company [3] (2011) “B5 Programme Will Strengthen CPO Price, Says MPIC Official.” BERNMA The Malaysian National News Agency. [Online]’ Available: http://galenet.galegroup.com/servlet/BCRC?srchtp=adv&c=1&ste=31&tbst =tsVS&tab=2&aca=nwmg&bConts=2&RNN=A250310952&docNum=A2 50310952&locID=upitt_main [4] (2011) “B5 Supply Nationwide Earliest By 2013, Says Dompok.” BERNMA The Malaysian National News Agency. [Online]’ Available:http://galenet.galegroup.com/servlet/BCRC?srchtp=adv&c=1&st e=31&tbst=tsVS&tab=2&aca=nwmg&bConts=2&RNN=A269329409&doc Num=A269329409&locID=upitt_main [5] N.N.A.N. Yusuf, S.K. Kamarudin, Z. Yaakub. (2011) “Overview on the current trends in biodiesel production.” [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0196890410005352 ACKNOWLEDGEMENTS We would like to take this time to thank all of those who helped us along the way to complete this conference paper to the best of our ability. Specifically the people we would like to thank are Janine Carlock, our writing center advisor; Katie Brown, the co-chair of our conference section; and Mark Jeffrey, the chair of our conference section. Thank you all so much for providing constructive criticism along the way. REFERENCES [1] M. Chin. (2011) “Biofuels in Malaysia: An analysis of the legal and institutional framework.” CIFOR. [Online]. Available: http://www.cifor.cgiar.org [2] Armaroli, Nicola (03/01/2011). "The Legacy of Fossil Fuels". Chemistry, an Asian journal(1861-4728), 6(3), p.768. [3] (2008) “America’s Most Polluted Cities Revealed.” [Online] Available: http://abcnews.go.com/Technology/Weather/story?id=4758772&page=1 [4] N. Hashimoto, Y. Ozawa, N. Mori, I. Yuri, T. Hisamatsu. (2008) “Fundamental combustion charcteristics of palm methyl ester (PME) as an alternative for gas turbines.” Fuel. [Online]. Available: http://www.elsevier.com/locate/fuel [5] J. Otera. (1993) “Transesterification.” [Online]. Available: http://pubs.acs.org/doi/abs/10.1021/cr00020a004?journalCode=chreay&qui cLinkVolume=93&quickLinkPage=1449&volume=93 [6] M. Balat and H. Balat. (2010) “Progress in biodiesel processing.” [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0306261910000255 [7] B.H. Hameed, L.F. Lai, L.H. Chin. (2008) “Production of biodiesel from palm oil using heterogeneous catalyst: an optimized process.” [Online]. Available: http://www.sciencedirect.com/science/article/pii/S0378382008003366 [8] “Alkali-Catalyzed Transesterification.” Chml Tech Ltd. [Online Article]. Available: http://www.chmltech.com/biodieseltech.htm [9] Z. Qiu, L. Zhao, L. Weatherley. “Process intensification technologies in 7